

Volkmann contracture (see the image below) is a permanent shortening of forearm muscles, usually resulting from injury, that gives rise to a clawlike deformity of the hand, fingers, and wrist. It is more common in children.
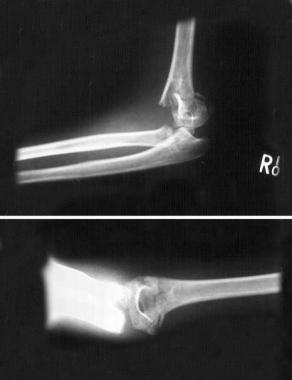 Volkmann contracture. Supracondylar fracture.
Volkmann contracture. Supracondylar fracture.
The clinical presentation includes the five Ps:
Additional useful findings are as follows:
See Clinical Presentation for more detail.
Radiographs of the humerus, elbow, and forearm are useful for assessing the amount of displacement of supracondylar fractures and combined radial and ulnar fractures. Nondisplaced supracondylar fractures rarely cause Volkmann contracture.
See Workup for more detail.
Initial treatment for Volkmann contracture consists of removal of occlusive dressings or splitting or removal of casts. Analgesics provide symptomatic relief in chronic cases.
Emergency fasciotomy is required to prevent progression to Volkmann contracture. Once contracture has occurred, treatment depends on the type of Volkmann contracture present, as follows:
Complications related to fasciotomy for Volkmann contracture include the following:
Both physical therapy and occupational therapy are vital to the improvement of range of motion and the return of function in patients with Volkmann contracture.
See Treatment for more detail.
NextVolkmann contracture (or Volkmann ischemic contracture) is a permanent shortening (contracture) of forearm muscles, usually resulting from injury, that gives rise to a clawlike deformity of the hand, fingers, and wrist. It is more common in children. A similar condition can occur in the foot.
In 1881, Richard von Volkmann attempted to ascribe irreversible contractures of the flexor muscles of the hand to ischemic processes in the forearm, in the belief that the problem was caused by massive venous stasis and simultaneous arterial insufficiency secondary to overly tight bandages.
In 1906, Hildebrand first used the term Volkmann ischemic contracture to describe the final result of any untreated compartment syndrome; he was also the first to suggest that elevated tissue pressure may be causally related to ischemic contracture.
In 1909, Thomas reviewed the 112 published cases of Volkmann contracture and found fractures to be the predominant cause. He also noted, however, that tight bandages, an arterial embolus, or arterial insufficiency could also lead to the problem. Since then, much has been learned about the etiologies of Volkmann contracture and, more important, about its preventive therapies.
In 1914, Murphy was the first to suggest that fasciotomy might prevent Volkmann contracture. He also suggested that tissue pressure and fasciotomy were related to the development of contracture.
During World War II and subsequently, many cases of Volkmann contracture occurred as a result of high-velocity gunshot wounds that caused fractures. Unfortunately, the arterial spasm accompanying the fracture was seen as the cause; therefore, more attention was directed to treating arterial spasm than to determining the need for fasciotomy.
Surgical exploration of the artery often led to reversal of an acute impending compartment syndrome—probably, it is now believed, because vascular surgeons were actually performing limited fasciotomies while they were exposing the vasculature. Appreciation of the importance of fasciotomy grew during the Vietnam War, and in 1967, Chandler and Knapp suggested that long-term results might have improved if the surgeons had included routine fasciotomy with arterial repairs.
Originally, most studies of ischemic contractures were focused on those of the upper extremity. In 1958, Ellis reported a 2% incidence of compartment syndrome with tibia fractures, and increased attention was paid to contractures involving the lower extremities.
Initially, the focus was on the anterior compartment of the leg, but the work of Seddon, Kelly, and Whitesides in the mid 1960s demonstrated the existence of four compartments in the leg and the need to decompress more than just the anterior compartment.[1, 2] Since then, compartment syndrome has been shown to affect many areas of the body, including the hands, feet, thighs, and buttocks.
Current research is aimed at reperfusion of the ischemic extremity. Some advocate the use of hyperbaric oxygen to improve tissue oxygenation and prevent further myonecrosis.[3] Early detection and prevention are still important in preventing severe disability. Frequent repeat examinations are required. Miniature transducer-tip catheters may allow continuous and accurate measurements of intracompartmental pressures.[4] Other noninvasive techniques for Volkmann contracture are currently under investigation.
The relevant anatomy of Volkmann contracture includes the superficial and deep flexor muscles. Superficial flexor muscles that may be involved in this process are as follows:
Deep flexor muscles that may be involved are as follows:
Volkmann contracture is usually seen in children with displaced supracondylar fractures of the humerus or forearm fractures.[5, 6, 7, 8] It results from severe injury to the deep tissues and muscles of the volar compartment secondary to increased compartmental pressures.[4, 9]
The following three types of Volkmann contracture have been described:
A variant of Volkmann ischemic contracture known as pseudo-Volkmann contracture has also been described in the literature. This condition results from tethering of the flexor digitorum profundus secondary to fractures of the ulna. It has been reported to occur 2 days to 16 years after closed reduction of fractures of the shafts of the radius and ulna; none of the patients described had nerve palsies or undue pain after reduction of the fractures.[10]
A routine check of the passive range of motion of all fingers immediately after closed reduction of radial or ulnar fractures is recommended. If muscle tethering is detected, repeat manipulation of the fracture is required to release the muscle. If this is unsuccessful, surgical release through a small incision should be attempted to normalize the length, excursion, and function of the flexor digitorum profundus. Function can be restored by untethering the muscle and its tendons from the ulnar fracture via early manipulation or late localized myotenolysis.
Any process that leads to increased compartmental pressure can lead to a compartment syndrome.[11] For example, a decrease in the compartment size with no change in the volume of the content results in increased pressure. This change can be secondary to closure of fascial defects, localized external pressure, or overly tight dressings.
Many processes lead to an increase in the volume of the content without a corresponding increase in the compartment size, thereby increasing pressure. Bleeding into a closed compartment can be due to a major vascular injury or to a congenital or acquired bleeding disorder.[12]
Increased capillary permeability can be due to exercise, burns, hypoalbuminemia, intra-arterial drugs, surgery, seizures and eclampsia, exercise, or trauma (without major vascular injury). Exercise, venous obstruction, and use of a long leg brace can lead to increased capillary pressure. Muscle hypertrophy or neoplastic processes can increase the volume of the compartment’s content and thus the intracompartmental pressure. Finally, infiltrated infusions are an iatrogenic cause of this state.
Overall, Volkmann contractures are rare, with an incidence of about 0.5%. In a review of 978 consecutive upper-extremity long-bone fractures in children admitted to the hospital over a 13-year period,[13] 33 patients had a supracondylar fracture, and three of these developed a compartment syndrome necessitating fasciotomy. In a subgroup analysis, nine children had ipsilateral displaced extension supracondylar humerus fractures or displaced forearm fractures. In this subgroup, the prevalence of Volkmann contracture was 33%.
Cubitus varus, or gunstock deformity, is the most common complication in Volkmann contracture. This results in the loss of the carrying angle of the upper extremity. Cubitus varus has been reported in as many as 25-60% of patients. The rate depends on the management approach. With the use of percutaneous pinning, the incidence of this complication has decreased to less than 10%.
With valgus or varus deformities in the coronal plane, remodeling is unlikely, if it is possible at all. Nerve injuries occur in 7% of cases, with common involvement of the radial, median, and ulnar nerves. Most deficits are seen at the time of injury. Fortunately, neurapraxias resolve with conservative management.[14] Motor function returns at 7-12 weeks, followed by the recovery of sensation, which may take more than 6 months.
Reportedly, 10% of children with supracondylar fractures temporarily lose the radial pulse. Fortunately, this loss is most often due to swelling and not to direct brachial artery injury. Reducing the fracture usually helps to return the arterial flow.
Clinical Presentation
Copyright © www.orthopaedics.win Bone Health All Rights Reserved