

Cerebral palsy is the leading cause of childhood disability affecting function and development. The incidence of the condition has not changed in more than 4 decades, despite significant advances in the medical care of neonates.
The magnetic resonance image (MRI) below illustrates the findings in a 16-month-old boy with cerebral palsy.
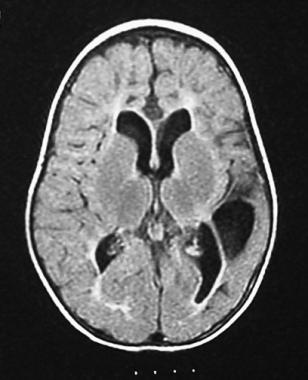 Magnetic resonance image (MRI) of a 16-month-old boy who was born at term but had an anoxic event at delivery. Examination findings were consistent with a spastic quadriplegic cerebral palsy with asymmetry (more prominent right-sided deficits). Cystic encephalomalacia in the left temporal and parietal regions, delayed myelination, decreased white matter volume, and enlarged ventricles can be seen in this image. These findings are most likely the sequelae of a neonatal insult (eg, periventricular leukomalacia with a superimposed left-sided cerebral infarct).
Magnetic resonance image (MRI) of a 16-month-old boy who was born at term but had an anoxic event at delivery. Examination findings were consistent with a spastic quadriplegic cerebral palsy with asymmetry (more prominent right-sided deficits). Cystic encephalomalacia in the left temporal and parietal regions, delayed myelination, decreased white matter volume, and enlarged ventricles can be seen in this image. These findings are most likely the sequelae of a neonatal insult (eg, periventricular leukomalacia with a superimposed left-sided cerebral infarct).
Signs of cerebral palsy include the following:
The patient’s overall gait pattern should be observed, and each joint in the lower and upper extremity should be assessed for signs of cerebral palsy, including the following:
See Clinical Presentation for more detail.
Laboratory studies
The diagnosis of cerebral palsy is generally made based on the clinical picture. There are no definitive laboratory studies for diagnosing the condition, only studies, including the following, to rule out other symptom causes:
Imaging studies
Cranial imaging studies to help evaluate brain damage and identify persons who are at risk for cerebral palsy include the following:
Other
Additional studies in cerebral palsy can include the following:
See Workup for more detail.
Management of abnormal movements
Numerous medications, including the following, may relieve the movement difficulties associated with cerebral palsy:
Surgery
Surgical treatments used in patients with cerebral palsy include the following:
See Treatment and Medication for more detail.
NextThe term cerebral palsy (CP) was originally coined more than a century ago and loosely translates as "brain paralysis." However, a precise definition has remained elusive because cerebral palsy is not a single diagnosis but an "umbrella" term describing nonprogressive brain lesions involving motor or postural abnormalities that are noted during early development.[9] Cerebral palsy has been described as follows[10] :
"A group of disorders of the development of movement and posture causing activity limitations that are attributed to non-progressive disturbances that occurred in the developing fetal or infant brain. The motor disorders of cerebral palsy are often accompanied by disturbances of sensation, cognition, communication, perception, and/or behavior and/or a seizure disorder."
Cerebral palsy is the leading cause of childhood disability affecting function and development. The brain lesions of cerebral palsy occur from the fetal or neonatal period to up to age 3 years. However, although insults to the brain after age 3 years through adulthood may manifest clinically as similar or identical to cerebral palsy, by definition, these lesions are not cerebral palsy. In addition, despite the fact that the lesion to the developing brain occurs before age 3 years, the diagnosis of cerebral palsy may not be made until after that time. Some authorities advocate not making a definitive diagnosis in selected cases until age 5 years or later. This approach allows the clinical picture to be clear and potentially allows exclusion of progressive diseases.[11, 12] In addition, some children have been diagnosed with cerebral palsy at an early age, only to have the symptoms resolve later.[13]
The magnetic resonance image (MRI) below illustrates the findings in a 1-year-old former preterm boy with cerebral palsy.
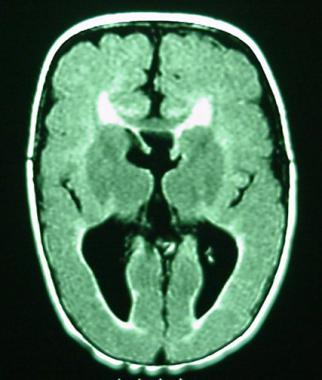 Magnetic resonance image (MRI) of a 1-year-old boy who was born at gestational week 27. The clinical examination was consistent with spastic diplegic cerebral palsy. Pseudocolpocephaly and decreased volume of the white matter posteriorly were consistent with periventricular leukomalacia. Evidence of diffuse polymicrogyria and thinning of the corpus callosum is noted in this image.
Magnetic resonance image (MRI) of a 1-year-old boy who was born at gestational week 27. The clinical examination was consistent with spastic diplegic cerebral palsy. Pseudocolpocephaly and decreased volume of the white matter posteriorly were consistent with periventricular leukomalacia. Evidence of diffuse polymicrogyria and thinning of the corpus callosum is noted in this image.
Approximately 30-50% of patients with cerebral palsy have mental retardation, depending on the type.[14, 15] However, because of oromotor, fine motor, and gross motor difficulties, communication in these patients may be impaired and expression of intellectual capacity may be limited. However, if cerebral palsy is approached in a multidisciplinary manner, with physical, occupational, and nutritional therapy to maximize rehabilitative efforts, patients can be more fully integrated academically and socially.
Approximately 15-60% of children with cerebral palsy have epilepsy, and epilepsy is more frequent in patients with spastic quadriplegia or mental retardation.
See also the following:
Cerebral palsy is classified according to resting tone and what limbs are involved (called topographic predominance). Spastic cerebral palsy, due to cortex/pyramidal tract lesions, is the most common type and accounts for approximately 80% of cases[12] ; this type of cerebral palsy is characterized by spasticity (velocity-dependent increase in tone), hyperreflexia, clonus, and an upgoing Babinski reflex.
Extrapyramidal or dyskinetic cerebral palsy comprises 10-15% of this disorder and is characterized more by abnormal involuntary movements. Ataxic cerebral palsy comprises less than 5% of cerebral palsy.
Many patients have characteristics of both spastic and extrapyramidal cerebral palsy. The typical types of cerebral palsy are as follows:
Functional classification systems generally divide patients into mild, moderate, and severe types (depending on functional limitations). Alternatively, patients may be categorized more comprehensively by their abilities and limitations, as was proposed by the World Health Organization in 2001. See International Classification of Functioning, Disability and Health (ICF).
Cerebral palsy is generally considered a static encephalopathy (ie, nonprogressive in nature). However, the clinical presentation of this condition changes as children and their developing nervous systems mature.
Advances in neonatal neurology continue to focus on potentially modifiable factors during the neonatal period that contribute to the development of cerebral palsy. In recent years, several studies have shown that antenatal magnesium sulfate given to mothers at risk for preterm delivery is associated with a significant reduction in the risk of cerebral palsy.[16, 17, 18] Many other studies focus on the role of excitable amino acids and their role in neurologic injury. The hope is that more can be done in the neonatal period to prevent the permanent neurologic deficit resulting in cerebral palsy.
In summary, no set rules exist as to where or when the brain injury can occur, and injury may occur at more than one stage of fetal brain development. Additionally, causes are multiple and potentially multifactorial, including vascular insufficiency, infection, maternal factors, or underlying genetic abnormalities. Regardless of the etiology, however, the underlying brain anomaly in cerebral palsy is static, although the motor impairment and functional consequences may vary over time. By definition, cases associated with underlying disorders of a progressive or degenerative nature are excluded when diagnosing cerebral palsy.
Cerebral palsy is restricted to lesions of the brain only; diseases specific to the peripheral nerves of the spinal cord (eg, spinal muscular atrophy, myelomeningocele) or to the muscles (eg, muscular dystrophies), although causing early motor abnormalities, are not considered cerebral palsy.
Major events in human brain development and their peak times of occurrence include the following[19] :
Cohort studies have shown an increased risk in children born slightly preterm (37-38 weeks) or postterm (42 weeks) compared with children born at 40 weeks.[20]
Given the complexity of prenatal and neonatal brain development, injury or abnormal development may occur at any time, resulting in the varied clinical presentations of cerebral palsy (whether due to a genetic abnormality, toxic or infectious etiology, or vascular insufficiency). For example, cerebral injury before the 20th week of gestation can result in a neuronal migration deficit; injury between the 26th and 34th weeks can result in periventricular leukomalacia (foci of coagulative necrosis in the white matter adjacent to the lateral ventricles); injury between the 34th and 40th weeks can result in focal or multifocal cerebral injury.
Brain injury due to vascular insufficiency depends on various factors at the time of injury, including the vascular distribution to the brain, the efficiency of cerebral blood flow and regulation of blood flow, and the biochemical response of brain tissue to decreased oxygenation.
The physical stress on premature infants and the immaturity of the brain and cerebral vasculature likely explain why prematurity is a significant risk factor for cerebral palsy. Before term, the distribution of fetal circulation to the brain results in the tendency for hypoperfusion to the periventricular white matter. Hypoperfusion can result in germinal matrix hemorrhages or periventricular leukomalacia. Between weeks 26 and 34 of gestation, the periventricular white matter areas near the lateral ventricles are most susceptible to injury. Because these areas carry fibers responsible for the motor control and muscle tone of the legs, injury can result in spastic diplegia (ie, predominant spasticity and weakness of the legs, with or without arm involvement of a lesser degree).
When larger lesions extend past the area of descending fibers from the motor cortex to involve the centrum semiovale and corona radiata, both the lower and upper extremities may be involved. Periventricular leukomalacia is generally symmetric and thought to be due to ischemic white matter injury in premature infants. Asymmetric injury to the periventricular white matter can result in one side of the body being more affected than the other. The result mimics a spastic hemiplegia but is best characterized as an asymmetric spastic diplegia. The germinal matrix capillaries in the periventricular region are particularly vulnerable to hypoxic-ischemic injury because of their location at a vascular border zone between the end zones of the striate and thalamic arteries. In addition, because they are brain capillaries, they have a high requirement for oxidative metabolism.
Many authorities grade the severity of periventricular hemorrhage-intraventricular hemorrhage using a classification system originally described by Papile et al in 1978 (see Periventricular Hemorrhage-Intraventricular Hemorrhage), as follows[21] :
At term, when circulation to the brain most resembles adult cerebral circulation, vascular injuries at this time tend to occur most often in the distribution of the middle cerebral artery, resulting in a spastic hemiplegic cerebral palsy. However, the term brain is also susceptible to hypoperfusion, which mostly targets watershed areas of the cortex (eg, end zones of the major cerebral arteries), resulting in spastic quadriplegic cerebral palsy. The basal ganglia also can be affected, resulting in extrapyramidal or dyskinetic cerebral palsy.
The clinical presentation of cerebral palsy may result from an underlying structural abnormality of the brain; early prenatal, perinatal, or postnatal injury due to vascular insufficiency; toxins or infections; or the pathophysiologic risks of prematurity. These may include preterm birth, multiple gestation, intrauterine growth restriction, male sex, low Apgar scores, intrauterine infections, maternal thyroid abnormalities, prenatal strokes, birth asphyxia, maternal methyl mercury exposure, and maternal iodine deficiency.[12, 13, 22, 23] .
Evidence suggests that prenatal factors result in 70-80% of cases of cerebral palsy. In most cases, the exact cause is unknown but is most likely multifactorial.[13]
Interpretation of the literature is limited by the lack of strict definitions in studies attempting to define a pathogenesis of cerebral palsy and the relatively small size of certain studies. An increasing amount of literature suggests a link between various prenatal, perinatal, and postnatal factors and this disorder. Epidemiologic studies suggest that prenatal factors play a predominant role in the etiology of cerebral palsy.
A Norwegian study involving children with cerebral palsy diagnosed before 5 years of age suggested that a low Apgar score at 5 minutes is associated with this order in all birth weights.[23] The prevalence of cerebral palsy was highest in children with a low birth weight; however, the odds ratio of this order being associated with a low Apgar score (< 4) was highest in normal weight children. Nonetheless, most children with cerebral palsy had Apgar scores higher than 4 at 5 minutes.[23]
Although preterm delivery is a well-established risk factor for cerebral palsy, a recent study suggests that postterm pregnancy at 42 weeks or later has been associated with an increased risk of this condition.[20]
The following maternal and prenatal risk factors statistically correlate with cerebral palsy:
The following factors during pregnancy also correlate statistically with cerebral palsy:
The apparent overrepresentation of cerebral palsy in multiple gestation pregnancies may relate more to the presence of prematurity or intrauterine growth retardation. Multiple gestations may not be an added risk for this disorder. The exception is when one twin dies; the surviving twin has a higher chance than a singleton of developing cerebral palsy.
The following perinatal factors are associated with an increased risk of cerebral palsy:
In 10% or less of cerebral palsy cases, birth asphyxia can be determined as the definitive cause. Even when birth asphyxia is thought to be associated clearly with cerebral palsy, abnormal prenatal factors (eg, intrauterine growth retardation, congenital brain malformations) may have contributed to perinatal fetal distress. Cases of cerebral palsy attributed to birth asphyxia must document clear evidence of acidosis, moderate to severe neonatal encephalopathy, restriction to spastic quadriplegia, dyskinetic or mixed types of cerebral palsy, and exclusion of other etiologies. Additionally, an intrapartum event must be suggested by a sentinel event, fetal heart rate changes, Apgar score less than 4 at 5 minutes, organ system damage related to tissue hypoxia, and early imaging abnormalities.[24]
Although Apgar scores provide a method for documenting cardiopulmonary and neuromotor status in the minutes following birth, low scores alone cannot be used as an indicator of birth asphyxia. Such scores may reflect circumstances unrelated to birth asphyxia, such as infections and other preexisting prenatal conditions.
The following postnatal factors may contribute to cerebral palsy:
Possible causes of cerebral palsy by type are discussed below.
Of all cases of cerebral palsy, 70-90% are congenital and 10-30% are acquired (eg, vascular, inflammatory, traumatic). In unilateral lesions of the brain, the vascular territory most commonly affected is the middle cerebral artery; the left side is involved twice as commonly as the right. Other structural brain abnormalities include hemi-brain atrophy and posthemorrhagic porencephaly. In premature infants, this may result from asymmetric periventricular leukomalacia.
In premature infants, spastic diplegia may result from parenchymal-intraventricular hemorrhage or periventricular leukomalacia. In term infants, no risk factors may be identifiable, or the etiology might be multifactorial.
Approximately 50% of spastic quadriplegic cerebral palsy cases are prenatal, 30% are perinatal, and 20% are postnatal in origin. This type is associated with cavities that communicate with the lateral ventricles, multiple cystic lesions in the white matter, diffuse cortical atrophy, and hydrocephalus.
The patient often has a history of a difficult delivery with evidence of perinatal asphyxia. Preterm infants may have periventricular leukomalacia. Full-term infants may have structural brain abnormalities or cerebral hypoperfusion in a watershed (ie, major cerebral artery end zone) distribution.
Dyskinetic (extrapyramidal) cerebral is associated with several unique etiologies. Historically, kernicterus, or acute neonatal bilirubin encephalopathy, was a major cause. With improvement in early management of hyperbilirubinemia, the vast majority cases of dyskinetic cerebral palsy are currently associated with presumed hypoxic ischemic injury rather than with hyperbilirubinemia.[25] In the absence of hypoxia, hyperbilirubinemia, or prematurity, the possibility of a metabolic or neurodegenerative disorder as a basis for this presentation must be considered.
Thus, dyskinetic cerebral palsy may be associated with hyperbilirubinemia in term infants or with prematurity without prominent hyperbilirubinemia. Hypoxia affecting the basal ganglia and thalamus may affect term infants more than preterm infants.
The incidence of cerebral palsy has not changed in more than 4 decades, despite significant advances in the medical care of neonates.
In developed countries, the overall estimated prevalence of cerebral palsy is 2-2.5 cases per 1000 live births.[26] The prevalence of this disorder among preterm and very preterm infants is substantially higher.[27, 28] In the developing world, the prevalence of cerebral palsy is not well established but estimates are 1.5-5.6 cases per 1000 live births. These figures may represent an underestimation because of a paucity of data, the lack of healthcare access, an overrepresentation of severe cases, and inconsistent diagnostic criteria.[12]
All races are affected by this disorder. Lower socioeconomic status[29] and male sex[12] may be increased risk factors for cerebral palsy.
With relation to age, the insult that gives rise to cerebral palsy occurs during immature brain development. According to most references, this initiating event can take place anytime between prenatal development and age 3 years. However, children are usually not diagnosed until after age 1 year, with the condition becoming identifiable as children fail to meet developmental milestones. Often, children who are older and are diagnosed as having cerebral palsy—as a result of having presenting symptoms or problems that are similar to those of cerebral palsy—should instead be labeled with the etiology of their brain injury (ie, traumatic brain injury secondary to a motor vehicle accident, stroke, metabolic condition, etc.
With appropriate therapeutic services, patients may be able to fully integrate academically and socially.
The morbidity and mortality of cerebral palsy relate to the severity of this condition and concomitant medical complications, such as respiratory and gastrointestinal difficulties. In patients with quadriplegia, the likelihood of epilepsy, extrapyramidal abnormalities, and severe cognitive impairment is greater than in those with diplegia or hemiplegia.
Cognitive impairment occurs more frequently in persons with cerebral than in the general population. The overall rate of mental retardation in affected persons is thought to be 30-50%. Some form of learning disability (including mental retardation) has been estimated to occur in perhaps 75% of patients. However, standardized cognitive testing primarily evaluates verbal skills and may result in the underestimation of cognitive abilities in some individuals.
In some studies, 25% of patients with cerebral palsy are unable to walk. However, many patients with this disorder (particularly those with spastic diplegia and spastic hemiplegia types) can ambulate independently or with assistive equipment. Thus, approximately 25% of children with cerebral palsy have mild involvement with minimal or no functional limitation in ambulation, self-care, and other activities. Approximately half are moderately impaired to the extent that complete independence is unlikely but function is satisfactory. Only 25% are so severely disabled that they require extensive care and are nonambulatory.
A prospective study of children has suggested that being able to sit by age 2 years is a good predictive sign of eventual ambulation. The suppression of obligatory primitive reflex activity by age 18-24 months was a sensitive indicator for distinguishing children who ultimately walked from those who were not expected to walk. Children who did not sit by age 4 years did not ambulate.
In patients with spastic quadriplegia, a less favorable prognosis correlated with a longer delay in the resolution of extensor tone. At times, hypertonicity and spasticity may improve or resolve over time in patients with cerebral palsy. Spasticity in patients with spastic quadriplegia can be more resistant even with services and orthopedic and rehabilitative intervention.
Patients with severe forms of cerebral palsy may have a significantly reduced life span, although this continues to improve with improved health care and gastrostomy tubes.[30] Patients with milder forms of this disorder have a life expectancy close to the general population, although it is still somewhat reduced.[31, 32, 33]
Cerebral palsy complications may affect multiple systems. For example, skin complications include decubitus ulcers and sores; orthopedic complications may include contractures, hip dislocation, and/or scoliosis.
Maintaining weight close to idea body weight is important for wheelchair-bound patients or those with ambulatory dysfunction. Nutrition consultation should be done early and periodically to ensure proper growth. Parents and medical professionals must keep on top of the potential nutritional difficulties in children with cerebral palsy. These patients are especially at risk of developing osteoporosis because of decreased weight bearing, so following their calcium intake is important.[34]
Gastrointestinal and nutritional complications include the following:
Dental problems also include enamel dysgenesis, malocclusion, and gingival hyperplasia. Malocclusion is twice as prevalent as in the normal population. The increased incidence of dental problems is often secondary to the use of medications, especially drugs administered to premature infants and antiepileptic agents.
Respiratory complications include the following:
Neurologic complications include the following:
Epilepsy occurs in 15-60% of children with cerebral palsy and is more common in patients with spastic quadriplegia or mental retardation. When compared with controls, children with cerebral palsy have a higher incidence of epilepsy with onset within the first year of life and are more likely to have a history of neonatal seizures, status epilepticus, polytherapy, and treatment with second-line anticonvulsants. Factors associated with a seizure-free period of at least 1 year include normal intelligence, single seizure type, monotherapy, and spastic diplegia.
Visual acuity decreases in premature infants because of retinopathy of prematurity with hypervascularization and possible retinal detachment.
Cognitive/psychologic/behavioral complications include the following:
Patients with cerebral palsy and their caregivers should be aware that oromotor dysfunction may require limitations in the texture of food and liquid, feeding only by gastrostomy or jejunostomy tube, supplemental feedings via gastrostomy or jejunostomy tube to increase energy intake, and aspiration precautions.
In addition, regular physical therapy and occupational therapy are crucial in these individuals. The goal should be to maximize the functional use of limbs and ambulation and to reduce the risk of contractures.
For patient education information, see Brain and Nervous System Center as well as Cerebral Palsy.
Clinical Presentation
Copyright © www.orthopaedics.win Bone Health All Rights Reserved