

Metastatic bone disease occurs when cancer spreads from a primary organ site to bone. The spine is the most common location of metastatic disease. See the image below.
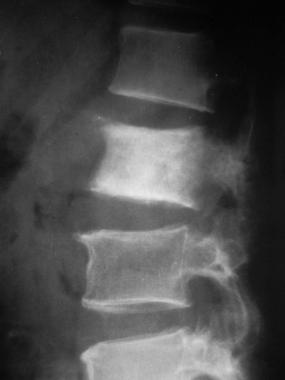 Lateral radiograph shows sclerotic metastasis of the L2 vertebra in a 54-year-old man with prostatic carcinoma.
Lateral radiograph shows sclerotic metastasis of the L2 vertebra in a 54-year-old man with prostatic carcinoma.
Pain is an important symptom of musculoskeletal metastases, but it is nonspecific. The pain pattern can be helpful if, in addition to being activity related, it is present at rest and at night, especially in patients older than 50 years. However, this pain pattern can be present in patients with osteomyelitis and Paget disease, and in these instances, it is also nonspecific.
Testing
Laboratory tests that can be used to aid in the diagnosis of metastatic bone disease include the following:
Imaging studies
The following radiologic studies may be used to evaluate metastatic bone disease:
Procedures
Biopsies should be obtained from any soft-tissue mass or, if no soft-tissue mass is present, from the most accessible bone in a mechanically safe area (eg, metaphysis vs diaphysis, acetabulum vs subtrochanteric femur).
In selected patients with metastatic disease of the spine, the following diagnostic procedures may be performed:
See Workup for more detail.
The life span of patients with metastatic bone disease is limited; thus, the goal of management needs to be centered on returning as much function as possible as rapidly as possible. Patients with metastatic bone disease are generally treated with surgery or radiation therapy.
Radiation therapy
Radiation therapy remains a primary therapeutic modality for the treatment of spinal metastasis, because nearly 95% of patients who are ambulatory at the start of radiation therapy remain so. Consequently, the possibility of regaining cord function once it is lost as a result of spinal metastasis is dismal. Therefore, such loss needs to be avoided by early diagnosis, treatment, and, if indicated, surgical intervention.
Surgery
The goals of surgical intervention for spinal surgery in patients with metastatic bone disease includes decreasing or eliminating pain, decompressing neural elements to protect cord function, and mechanically stabilizing the spine.[1, 2] Anterior or posterolateral decompression, combined with anteroposterior reconstruction, may be used in the following:
Vertebroplasty, in which polymethylmethacrylate is percutaneously introduced, may be a minimally invasive treatment alternative for patients with 1- or 2-level vertebral body compression fractures.[3]
For the management of long bone metastatic disease accompanied by an impending or completed fracture, open internal fixation is usually the preferred method of treatment. Stabilization with a locked intramedullary device followed by radiation therapy to the entire bone as soon as the surgical wounds have healed is preferred.[4]
Devices and/or procedures used in the surgical fixation of long bones include the following:
Pharmacotherapy
Medications used in the treatment of metastatic bone disease include the following:
See Treatment and Medication for more detail.
NextThe orthopedic surgeon has two major tasks to perform when treating patients who develop bone metastases.[1] The first task is to biopsy a bony lesion of unknown origin, which may be found during evaluation/staging studies or as a result of a patient's symptoms. (See Workup.)
The orthopedic surgeon's second task is to manage the stabilization of impending or already completed pathologic fractures of bones in a critical area, such as an upper or lower extremity, the pelvis, or the spine. In one study of patients with breast carcinoma, 19% of the patients developed a pathologic fracture or hypercalcemia as the first sign that the carcinoma had spread to bone. Moreover, 10% of the patients suffered spinal cord compression, and 9% of them experienced bone marrow failure. (See Prognosis and Treatment.)
In patients with bone metastases, it is important to develop strategies that emphasize maintenance of function, including ambulation, for the remainder of these patients’ lives and to intervene when possible before a fracture occurs. The morbidity and mortality rates associated with metastatic bone disease are greater when intervention is delayed. (See Prognosis and Treatment.)
In females, the breasts and lungs are the most common primary disease sites; approximately 80% of cancers that spread to bone arise in these locations.[5, 6, 7, 8, 9] In males, cancers of the prostate and lungs make up 80% of the carcinomas that metastasize to bone.[10] The remaining 20% of primary disease sites in patients of both sexes are the kidney, gut, and thyroid, as well as sites of unknown origin. (See Etiology and Pathophysiology.)
Previously, the two main theories of how tumor cells metastasize and grow in bones were Paget's fertile soil hypothesis and Ewing's circulation theory. However, a substantial amount of work has more clearly defined the metastatic process to bone. Bone metastases occur in a predictable distribution. In order of frequency, the most common locations include the following:
The red marrow theory, combined with knowledge about the cytokine stimulation of metastases, provides an excellent explanation of how this distribution occurs.
Metastases distal to the knee and elbow are extremely uncommon, but approximately 50% of these acral metastases are secondary to primary lung tumors. Carcinomas, such as those of the breast and prostate, rarely exhibit such a distinct pattern.
In 1995, Mundy and Yoneda described the cellular events necessary for the success of the metastatic process, including the attachment of tumor cells to the basement membrane, the production of proteolytic enzymes by tumor cells (such as metalloproteinases, which are enzymes that disrupt basement membranes), and the migration of tumor cells through the basement membranes into surrounding tissue, especially the arteriolar network.[11]
Cells from the primary site must, through the process of neovascularization or through migration to the nearest blood vessel, attach to the basement membrane of the vessel wall and produce proteolytic enzymes that disrupt the basement membrane.
The cells then migrate through the basement membrane and float away in the bloodstream to a distant site. The process through which these tumor cells are attracted to a specific site in the body is not completely clear, although type I collagen, a byproduct of bone resorption, has been shown to be a chemotactic factor that attracts tumor cells to bone.
If they survive the journey to the distant site, the tumor cells attach to the basement membrane of the vessel wall using proteolytic enzymes (integrins/cadherins). After disrupting the receptor site basement membrane, they migrate into the substance of the distal host tissue. Producing chemotactic factors, as well as RANK ligand (a transmembrane or soluble protein essential for the formation, function, and survival of osteoclasts), these cells stimulate osteoclast activity, causing bone resorption and leading to the formation of pockets or holes in the bone in which the tumor cells grow.
Another important substance that stimulates bone resorption is parathyroid hormone ̶ related peptide (PTHrP). This substance is expressed by breast carcinoma cells, as well as by oat cell tumors of the lung, and is a potent stimulant of osteoclasts. In 1996, Guise and colleagues reported elevated PTHrP levels in the bone marrow plasma (as compared with serum plasma levels) in rats with tumors.[12]
An interesting concept, reported in 1995 by Mundy and Yoneda, is that myeloma cells are especially adapted to producing bone destruction through direct stimulation of osteoclasts.[11] During the resorption process, the osteoclasts release interleukin-6, which is a major regulatory factor in the growth of myeloma cells. Additional myeloma cells further stimulate increased osteoclastic production in a continuous feedback mechanism. This enhances survival of the tumor cells and further destruction of the bone.[11]
Approximately 1.2 million patients present with cancer each year in the United States. Of these, approximately 600,000 persons have metastases to bone. In contrast, 2,700 patients per year develop primary bone sarcoma.
The age range of patients with sarcoma is different from that of individuals with carcinoma of bone; most metastatic bone lesions occur in adults older than 50 years, while most sarcomas occur in adolescents or young adults (<30 years). Therefore, a bone-occupying mass in an adult is much more likely to be a focus of metastatic carcinoma than to be a primary sarcoma of bone. However, in a patient with a bone lesion with no documentation of metastatic disease, caution is warranted to ensure the correct diagnosis.
In general, once skeletal metastases are present, patient survival is dramatically shortened. For example, the 5-year overall survival rate for people with prostate cancer is 93%, but once skeletal metastases are present, the average survival time is only 29 months. However, patients are surviving and remaining active for longer periods as treatment protocols improve. These factors make the orthopedic surgeon's task in prophylactic or reconstructive surgery more challenging.[13]
In addition, perioperative complications occur more frequently among patients with skeletal metastases; the perioperative mortality rate in this population is approximately 8%, and the perioperative infection rate is approximately 4% (although the infection rate is higher at previously irradiated sites).
Most patients with metastatic bone disease survive for 6-48 months. In general, patients with breast and prostate carcinoma live longer than do persons with lung carcinoma.[14, 15] Patients with renal cell or thyroid carcinoma have a variable life expectancy.
Kirkinis et al studied 462 patients presenting with metastatic bone disease to the extremities or pelvis who underwent orthopedic treatment.[16] Overall surival rates were 45% at 1 year, 29% at 2 years, and 13% at 5 years. Preoperative hemoglobin was found to be an independent predictor of better survival; lung histotype, age, pathologic fracture, and previous combined therapy were negative predictors of survival.
Clinical Presentation
Copyright © www.orthopaedics.win Bone Health All Rights Reserved