

Carpal tunnel syndrome (CTS) is the most commonly diagnosed and treated entrapment neuropathy. The syndrome is characterized by pain, paresthesia, and weakness in the median nerve distribution of the hand. Surgical and nonsurgical treatments exist that can produce excellent outcomes for patients.
In 1854, Sir James Paget first reported median nerve compression at the wrist following a distal radius fracture.[1] In 1880, James Putnam presented the first series of patients with pain and paresthesia in the median nerve distribution of the hand.[2] In 1913, Pierre Marie and Charles Foix described the pathology of median nerve compression underneath the transverse carpal ligament (TCL).[3] In 1933, Sir James Learmonth reported the first TCL release to treat median nerve compression at the wrist.[4] Since these early reports, much work has described the signs and symptoms of CTS, as well as its treatments.
For patient education resources, see Carpal Tunnel Syndrome.
In February 2016, the American Academy of Orthopaedic Surgeons (AAOS) published an evidence-based clinical practice guideline for the management of carpal tunnel syndrome (CTS), which included the following recommendations[5] :
The carpal canal is a fibro-osseous tunnel at the wrist through which nine flexor tendons and the median nerve pass.[6] The carpal bones define the dorsal aspect of the carpal canal and are shaped in a concave arch. The palmar aspect of the carpal canal is defined by the transverse carpal ligament (TCL), which bridges the two sides of the carpal arch. Intrinsic and extrinsic ligaments of the wrist and hand further stabilize the carpal bones. The carpal canal is narrowest at the level of the hook of the hamate, where the canal averages 20 mm in width.
The TCL attaches to the scaphoid tuberosity and trapezial crest on the radial side of the wrist, as well as to the pisiform and hook of the hamate on the ulnar side of the wrist (see the image below). The TCL is 1.5 mm thick and 21.7 mm in length on average. Proximally, it is a continuation of the antebrachial fascia in the forearm, and distally, it attaches to the fibers of the midpalmar fascia.
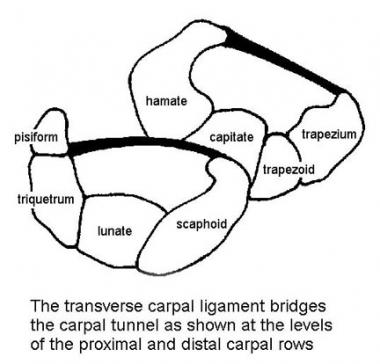 Cross sections of carpal canal at levels of proximal and distal carpal rows. Transverse carpal ligament bridges carpal tunnel and is under tension.
Cross sections of carpal canal at levels of proximal and distal carpal rows. Transverse carpal ligament bridges carpal tunnel and is under tension.
The TCL is under tension and helps to maintain the carpal arch. It serves as a retinacular pulley for the flexor tendons. Cutting the TCL increases the volume of the carpal canal. Cutting the TCL has also been postulated to alter the kinematics of the carpus, risk bowstringing of the flexor tendons, and decrease grip strength.
A combination of the lateral (C6-7) and medial (C8-T1) cords of the brachial plexus forms the median nerve. At the wrist and into the palm, the median nerve divides into terminal motor and sensory branches, with some anatomic variability. The variability is caused in part by the branching point of the recurrent motor branch (ie, extraligamentous or subligamentous).
An extraligamentous pattern, with a branching point distal to the TCL, is the most common. The recurrent motor branch can also divide from the median nerve underneath the TCL in a subligamentous fashion; it can then either wrap around the distal end of the TCL or pass directly through the TCL to innervate the thenar muscles. Other less common patterns, such as a branch point proximal to the TCL, exist as well. These variations can have major surgical implications.
The ulnar nerve is the other major motor and sensory nerve of the hand. The ulnar nerve does not pass through the carpal canal but instead goes through the Guyon canal, which is located adjacent to the carpal canal, at the wrist. Division of the TCL will change the morphology of the Guyon canal from triangular to ovoid.
The pathophysiology of CTS is typically demyelination. In more severe cases, secondary axonal loss may be present. The most consistent findings in biopsy specimens of tenosynovium from patients undergoing surgery for idiopathic CTS have been vascular sclerosis and edema.[7] Localized amyloid deposition in the tenosynovium also has been reported in persons with idiopathic CTS. Inflammation, specifically tenosynovitis, is not part of the pathophysiologic process in chronic, idiopathic CTS.
The etiology of CTS is multifactorial, with local and systemic factors contributing to varying degrees. Symptoms are a result of median nerve compression at the wrist, with ischemia and impaired axonal transport of the median nerve across the wrist.[8] Compression results from elevated pressures within the carpal canal.
Elevated pressures can develop within the carpal canal even though the canal is not a separate, closed compartment within the upper extremity. Direct pressure or a space-occupying lesion within the canal can increase pressure on the median nerve and produce CTS. Fracture callus, osteophytes, anomalous muscle bodies, tumors, hypertrophic synovium, and infection, as well as gout and other inflammatory conditions, can produce increased pressure within the carpal canal. Extremes of wrist flexion and extension also elevate pressure within the canal.
Compression of a nerve affects intraneural blood flow.[9, 10, 11] Pressures as low as 20-30 mm Hg retard venular blood flow in a nerve. Axonal transport is impaired at 30 mm Hg. Neurophysiologic changes manifested as sensory and motor dysfunction are present at 40 mm Hg. Further pressure increases lead to increasing sensory and motor block. At 60-80 mm Hg, intraneural blood flow ceases completely. In one study, the carpal canal pressures in patients with CTS averaged 32 mm Hg, compared with only about 2 mm Hg in control subjects.[9]
The double-crush syndrome, in which there is pressure on the median nerve at a second site (remote from the wrist), can further lower the median nerve's pressure threshold for producing symptoms of CTS. If a nerve is compressed at multiple sites, traction within the nerve with joint motion may be produced. In addition to pressure, traction or stretch has been demonstrated to produce alterations in intraneural circulation. Elongation of only 8% can impair venular flow, and all intraneural microcirculation can cease at 15% nerve elongation.
Many systemic conditions are strongly associated with CTS. These conditions may directly or indirectly affect microcirculation, pressure thresholds for nerve conduction, nerve cell body synthesis, and axon transport or interstitial fluid pressures. Perturbations in the endocrine system, as observed in individuals with diabetes and hypothyroidism and in women who are pregnant, are linked to CTS. Conditions affecting metabolism (eg, alcoholism, renal failure with hemodialysis, mucopolysaccharidoses) also are associated with CTS.
The international debate regarding the relation between CTS and the performance of repetitive motion and work is ongoing.[12, 13] The Occupational Safety and Health Administration (OSHA) has adopted rules and regulations regarding cumulative trauma disorders. Occupational risk factors — repetitive tasks, force, posture, and vibration — have been cited. However, the American Society for Surgery of the Hand has stated that the current literature does not support a causal relation between specific work activities and the development of diseases such as CTS.
Psychosocial and socioeconomic issues are increasingly being studied. In a study of risk factors for CTS in women, the greatest risk factor was found to be a previous history of another musculoskeletal complaint.[14] Perceptions of health and tolerance to pain also may influence the development of CTS.
The etiology of CTS and its relation to the workplace will continue to be better understood in the coming decades. It is already apparent that the etiology of CTS is multifactorial. Although work-induced repetitive trauma may not be the major cause of CTS, it may contribute in some way.
CTS is common in the general population.[15] It has previously been reported with acute onset after trauma to the wrist; it has also been detailed as a gradual progression of symptoms typically occurring in women who are in the late middle-aged years of life. A new population at risk has been reported to be industrial workers whose hands and wrists are subjected to repetitive motion and trauma.[12, 13]
Controversy exists regarding the clinical and electrophysiologic findings necessary to diagnose CTS. Despite this controversy, several surveys have been conducted to determine the prevalence of CTS in the general population. In the Netherlands, the prevalence of undetected CTS was 5.8% in women and 0.6% in men.[15] In Sweden, the overall prevalence of CTS in the population was 2.7%. These prevalence rates were based on clinical and electrophysiologic criteria and probably represent minimum prevalence rate estimates.
In a Mayo Clinic study by Gelfman et al, temporal trends in CTS were assessed for incidence, surgical treatment, and lost time at work,[16] using Olmsted County residents as the study population. Between 1981 and 2005, a total of 10,069 residents were found to have been diagnosed with CTS (491 per 100,000 person years for women; 258 per 100,000 person years for men; 376 per 100,000 person-years combined).
Adjusted annual rates increased from 258 per 100,000 in 1981-1985 to 424 in 2000-2005. The average annual incidence of carpal tunnel release surgery was 109 per 100,000, and that for work-related CTS was 11 per 100,000.[16] According to the authors, the increase seen in this population corresponds to a national epidemic of CTS cases resulting in lost work days that began in the mid-1980s and lasted through the mid-1990s, but the cause for the increase is not yet clear.
Wolf et al studied the diagnosis of CTS in the United States military population from 1998 to 2006 and found the incidence to be 3.98 per 1000 person-years (compared with 1.5-3.5 per 1000 person-years in other regional or working-group populations studied).[17] In this military study, females had a significantly higher incidence of CTS than males, with an adjusted incidence rate ratio of 3.29.
CTS incidence was found to increase with age, with the age group 40 years or older having a significantly higher incidence.[17] Additionally, military rank was found to be an independent risk factor for CTS, with rates higher in senior officer and enlisted groups, suggesting that occupational requirements have an effect on CTS within the military.
Lasting relief of pain, numbness, and paresthesia can be expected in more than 90% of patients with CTS who are treated by means of open or endoscopic carpal tunnel release; patient satisfaction is high. The endoscopic technique is associated with a shorter interval before the patient returns to work and with less incisional pain.[18] The primary reason for a poor result is an error in diagnosis.
Jarvik et al compared surgical treatment (57 patients) with multimodality nonsurgical treatment (hand therapy and ultrasound; 59 patients) for CTS without denervation.[19] Analyses showed a significant 12-month adjusted advantage for surgery in function and symptoms; there were no clinically important adverse events and no surgical complications. According to the authors, symptoms in both groups improved, but surgical treatment led to better outcome than nonsurgical treatment did.
Pomerance et al compared direct costs and results for surgical versus nonsurgical care in 120 patients with electrodiagnostically proven CTS.[20] Follow-up averaged 13 months for the nonsurgical group and 12 months for the surgical group, with 32 patients in the former group electing to have surgery during the follow-up period. Cost averaged $3335 in the nonsurgery group and $3068 in the surgery group. The authors concluded that surgery should be considered as initial treatment of electrodiagnostically confirmed CTS because it provides symptom resolution with a favorable cost profile.
In a study of 950 open carpal tunnel procedures performed in 826 patients (age range, 21-100 years) at a high-volume orthopedic surgery center, Cagle et al found that the subjects showed significant improvements in symptom severity at 2 weeks after surgery and in functional severity at 6 weeks.[21]
In this study, the postoperative improvement was not affected by documented medical comorbidities, though patients with a medical comorbidity had a slightly higher (but statistically insignificant) risk of negative postoperative endpoints.[21] Improvement was slower in diabetic patients but was essentially equivalent at 6 weeks. In patients with workers' compensation insurance, symptom severity and hand function were significantly worse at baseline, 2 weeks, and 6 weeks, but no significant differences remained at 3 months.
Clinical Presentation
Copyright © www.orthopaedics.win Bone Health All Rights Reserved