

Distal radius fractures account for approximately 15% of all fractures in adults. A thorough understanding of the pathophysiology and treatment of distal radius fractures is important because these injuries are not limited to just the elderly population. High-energy trauma to the distal radius in younger adults is becoming more prevalent, and long-term functional results are unclear. With an aging patient population that is increasingly active, these common injuries must be evaluated thoroughly and treated adequately.[1, 2, 3, 4, 5, 6]
In a study by Clayton et al, a high correlation was identified between bone mineral density and the severity of distal radius fractures. In patients with osteoporosis, the probability of early instability was 43%; the probability of late carpal malalignment, 39%; and the probability of malunion, 66%. In patients with osteopenia, the probability of early instability was 35%; the probability of late carpal malalignment, 31%; and the probability of malunion, 56%. These findings compared with a 28% probability of early instability, a 25% probability of late carpal malalignment, and a 48% probability of malunion in patients with normal bone mineral density.[7]
Koenig et al evaluated whether early internal fixation or nonoperative treatment is preferred for displaced, potentially unstable distal radial fractures with initial adequate reduction. They found that internal fixation with a volar plate provided a higher probability of painless union for potentially unstable distal radius fractures. In most cases, long-term gain in quality-adjusted life years outweighed the short-term risks of surgical complications, making early internal fixation the preferred treatment. In patients older than 64 years, however, nonoperative treatment may be preferred because of lower disutility for malunion and painful malunion outcome states.[8]
In postmenopausal women, detailed bone structure and strength measurements provide insight into the pathogenesis of forearm fracture, but femoral neck area bone mineral density (aBMD) provides adequate measurement for routine clinical risk assessment, according to Melton et al. Fracture cases had inferior bone density, geometry, microstructure, and strength. The factor-of-risk was 15% worse in patients with forearm fracture.[6] See also the Fracture Index WITH known Bone Mineral Density (BMD) calculator.
For excellent patient education resources, visit eMedicineHealth's First Aid and Injuries Center. Also, see eMedicineHealth's patient education article Broken Arm.
NextThe typical mechanism of a dorsally displaced distal radius fracture is a fall on an outstretched hand. This type of injury results in tensile forces across the volar surface (compression side), compressive forces on the dorsal surface (tension side), and supination of the distal fracture fragment. In the young adult, distal radius fractures are often caused by high-energy trauma. In the elderly patient, low-energy trauma, such as a fall from a standing height, can result in this injury.
Compression and torsion across the articular surface can cause various patterns of intra-articular displacement. Dorsal and palmar shear fractures of the medial complex are examples of compression applied to specific locations. Radial styloid fractures can be due to compression and/or torsion.
The dorsal metaphysis of the distal radius is subject to tensile and compressive forces during routine forearm activities. The volar surface transmits higher compressive forces. Stable reduction of the distal radius fracture requires that this biomechanical relationship be reestablished. Accordingly, the volar buttress must be addressed first in unstable volar fractures (eg, volar Barton fracture, distal radius fracture with significant volar comminution). In the presence of volar comminution or inherently unstable volar vertical shear fractures, the key to stable fracture reduction is to create a solid volar buttress either by accurate reduction of large volar metaphyseal fragments or by placement of a volar buttress plate. Once volar stability is restored, the dorsal metaphyseal fragments can be reduced against the stable volar buttress.
Restoration of volar stability also has important radiocarpal implications because the stout radiocarpal ligaments are attached to the volar surface. Therefore, volar integrity is critical for the following reasons: (1) it allows reduction of dorsal metaphyseal fragments against a stable volar buttress and (2) it prevents possible radiocarpal instability.
Studies have shown that distal radius fractures often are associated with tears of the triangular fibrocartilage complex (TFCC), scapholunate ligament, and lunotriquetral ligament.[9, 10] Geissler et al found that intracarpal soft-tissue injuries occurred most frequently with fractures involving the lunate facet.[9] The lunate facet and its strong ligamentous attachments with the proximal carpal row and ulnar styloid form the medial complex of the distal radius as described by Melone and shown in the image below.[11] The carpus almost always is displaced with the palmar and/or dorsal lunate facet die-punch fragment of the distal radius because of the exceptionally strong ligaments of the medial complex.
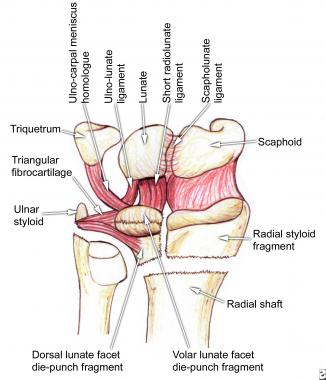 The medial complex, as described by Melone, consists of the lunate facet and its ligamentous attachments, especially the strong volar ligaments. Displacement of the medial complex has important functional implications.
The medial complex, as described by Melone, consists of the lunate facet and its ligamentous attachments, especially the strong volar ligaments. Displacement of the medial complex has important functional implications.
Scapholunate dissociation can occur with severely displaced distal radius fractures, and the lunate displaces with the medial complex (lunate fossa), while the scaphoid remains with the radial styloid. The scapholunate diastasis usually corrects with reduction of the medial complex. Most of the disrupted soft tissues of the scapholunate articulation can heal with a period of immobilization, and Melone had no cases of subsequent chronic carpal instability.[11]
Scapholunate and lunotriquetral ligament injuries can occur in minimally displaced extra-articular fractures and in severely comminuted intra-articular fractures. The presence of central perforations of the scapholunate ligament and tears of the short radiolunate ligaments has important implications. Although these injuries do not result in scapholunate instability, Richards et al found false findings on arthrograms in 8% of patients in whom arthrography rather than arthroscopy was used for diagnosis.[10] With arthroscopy, it is difficult to evaluate injury to the volar extrinsic ligaments, including the radioscaphocapitate and long radiolunate ligaments, because these ligaments may be pulled taut with the longitudinal traction necessary for entry of the arthroscope.[9]
Distal radius fractures characterized by shortening and dorsal angulation are more likely to have a TFCC disruption, but preoperative radiographs have no predictive value in identifying specific interosseus ligament injuries. Intra-articular and extra-articular distal radius fractures commonly are associated with ligamentous injuries and tears of the radial aspect of the TFCC; however, disruption of the ulnar insertion of the TFCC is uncommon. The fact that certain intra-articular fracture patterns are associated with fewer TFCC injuries emphasizes the role that the TFCC plays in force dissipation and stability after a distal radius fracture.[10]
In general, the authors do not treat carpal ligament injuries, including the TFCC, that are associated with distal radius fractures that do not show visible deformities on plain radiographs. The authors believe that, with accurate fracture reduction, the ligaments heal during the postoperative or postreduction immobilization period. However, whenever an external fixator is applied, it must be used for neutralization only because excessive traction can displace or complete undiagnosed partial carpal ligament tears.
Burstein states that a classification system must suggest a method of treatment and provide a reasonably precise estimation of the outcome of that fracture. Furthermore, a classification system must have intraobserver repeatability and interobserver reliability.[12]
Although the Frykman system for classification of distal radius fractures has been used extensively in the medical literature, this classification fails to identify the direction and extent of fracture displacement.[13] As a result, other classification tools have been developed, such as the Association for the Study of Internal Fixation (AO/ASIF), Melone, and Mayo systems. These systems classify the fractures on the basis of 4 distinguishing characteristics: extent of comminution, radiographic appearance or magnitude of displacement, articular joint involvement, and mechanism of injury.[13]
Andersen et al compared the Frykman, Melone, Mayo, and AO/ASIF classification systems and concluded that a low degree of intraobserver and interobserver agreement exists in each of these 4 systems. Consequently, the use of any of these classifications as the primary method to determine treatment and outcome of treatment is not warranted.[14]
Andersen et al also state, "Some orthopaedists have expressed concern, especially in training programs, that more effort is spent trying to memorize classification systems for a number of fractures, rather than truly understanding the fracture mechanics or the factors that have significant bearing on prognosis or treatment."[14]
Despite the negative aspects of the various tools for classifying distal radius fractures, the AO/ASIF system reached the "substantial level" for both interobserver and intraobserver agreement when these tools were reduced to 3 broad fracture categories[14] : extra-articular, partial articular, and complete articular. These 3 general fracture categories are incorporated in the classification system that the authors prefer and propose in the Table below.
[#table]
Table. Classification and Treatment Guidelines for Distal Radius Fractures. (Open Table in a new window)
Fracture Treatment* A: Extra-articular Stable (nondisplaced or reduced) CR, splinting Unstable (displaced)†In looking at the radiographic characteristics of intra-articular fractures, the Melone 4-part pattern seems to be fairly reproducible. The basic fragments of the Melone 4-part pattern consist of the radial styloid, dorsal lunate facet die-punch fragment, palmar lunate facet die-punch fragment, and the radial shaft, as shown in the image below. Various combinations of these basic fragments of the Melone 4-part pattern are manifested consistently in intra-articular distal radius fractures. In addition to variability of fragment displacement, variability of comminution of each individual component fragment also exists.
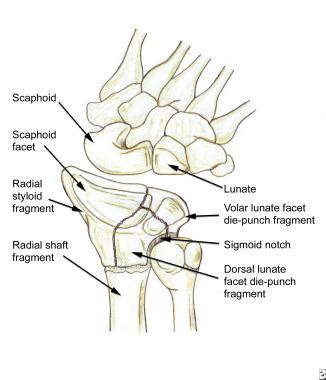 The basic fragments of the Melone 4-part pattern consist of the radial styloid, dorsal lunate facet die-punch fragment, volar lunate facet die-punch fragment, and radial shaft. Note that displacement of the dorsal and/or volar lunate facet die-punch fragments also alters the anatomy of the sigmoid notch articular surface; thus, it has important consequences for forearm pronation and supination.
The basic fragments of the Melone 4-part pattern consist of the radial styloid, dorsal lunate facet die-punch fragment, volar lunate facet die-punch fragment, and radial shaft. Note that displacement of the dorsal and/or volar lunate facet die-punch fragments also alters the anatomy of the sigmoid notch articular surface; thus, it has important consequences for forearm pronation and supination.
Of historical interest, the Melone 4-part pattern can be viewed as the summation of the eponymous Colles, Smith, Barton, and chauffeur fractures.[16] The volar and dorsal vertical shear fractures (Smith II/volar Barton fracture and dorsal Barton fracture, respectively) have classically been described as partial articular injuries involving the lunate facet of the distal radius. These injuries include volar or dorsal carpal displacement because of the important extrinsic radiocarpal ligaments that attach to the lunate facet. As such, displaced volar and dorsal vertical shear fractures (Barton/Smith fractures) have the same biomechanical implications and treatment methods as displacement of Melone palmar and dorsal lunate facet die-punch fragments, respectively.
The authors' selection of treatment is based consistently on the particular configuration and displacement of the Melone fracture components. Because the goal of a good classification system is to define reproducible clinical characteristics that can guide treatment selection, the authors believe that their treatment algorithm can also serve as a practical classification system for distal radius fractures. This system is presented in the Table and further explained in Treatment.
If a dorsal lunate facet die-punch component does not have significant dorsal metaphyseal comminution, it can be reduced against the intact volar surface and stabilized by transfixing percutaneous pins.
Inherent stability is restored with good dorsal cortical apposition. In highly comminuted dorsal fractures in which contact with the dorsal metaphyseal cortex is lost, inherent dorsal stability is established by using bone grafts as void fillers, in combination with external fixation, to maintain neutral tension in the dorsal aspect.
In the presence of unstable volar fragments, the anterior cortex cannot serve as an adequate anterior buttress against which the dorsal fragments can be reduced. In these instances, the authors routinely add a volar plate to stabilize the volar distal cortex (see the image below). Thus, in cases with combined dorsal and volar instability, the dorsal fragments are treated as outlined above, but the volar cortex is reconstructed first with a volar buttress plate.
 Postsurgical lateral radiograph shows a good reduction of the fracture with a volar buttress plate.
Postsurgical lateral radiograph shows a good reduction of the fracture with a volar buttress plate.
If a displaced radial styloid component is present, it is reduced manually and, if required, stabilized with 2 parallel radial styloid pins. Open reduction of the radial styloid may be necessary if closed reduction (CR) is not successful, and, in the presence of comminution and instability, volar radial plating of the radial styloid is an effective treatment option. Other treatment modalities, such as the use of small lateral buttress plates and clips are currently being investigated. Pronation or, more commonly, supination deformity of the radial styloid must be corrected. The use of intra-operative fluoroscopy is helpful in identifying rotation of the styloid fragment.
Thus, the classification system the authors prefer is a modification of the AO/ASIF and Melone systems that incorporates the various treatment principles described above. The classification system is derived from rational treatment-based options that the authors believe reflect the physiologic differences in each fracture pattern. The Table represents the authors' classification system and a practical guide for treatment of distal radius fractures.
On presentation, history should include the patient's pertinent past medical history, occupation, hand dominance, mechanism of injury, and treatment history. The patient's dependence on the extremity for occupational needs and activities of daily living greatly affects later decision-making.
Start the physical examination proximally at the shoulder and continue distally to include the elbow, wrist, and hand. Visually inspect the wrist and note the presence or absence of open wounds, swelling, and deformity. Pain may limit manual examination and range of motion (ROM) of the injured wrist, but investigate other proximal injuries because they may alter the treatment plan. An adequate neurologic and vascular examination with particular attention to the median nerve is essential. Tests for compartment syndrome also must be performed carefully.
Graham proposed the following radiographic criteria for acceptable reduction of a distal radius fracture[17] :
Articular incongruity less than 2 mm of the sigmoid notch of the distal radius is another critical radiographic parameter.
Radial shortening has been shown to be the most important reduction parameter because of its impact on the radiocarpal joint, DRUJ, and, ultimately, functional outcome.[11, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27] Palmer and Werner even found that minor axial shortening of 2 mm can alter the contact forces across the entire wrist joint.[24] Complications of radial shortening include increased pressure on the TFCC, pain caused by ulnocarpal impingement, increased lunate contact area, an increase of approximately 40% of the ulnar axial load, decreased and/or painful pronation or supination, and decreased grip strength.[18, 24, 28, 29]
Radial inclination less than 15° can cause an appearance of radial deviation of the wrist and changes the load distribution between the scaphoid and the lunate.[24, 30]
Most wrist fractures usually result in a dorsal tilt of the distal radius articular surface in the sagittal plane. Fernandez found that patients with a dorsal tilt more than 25° usually become symptomatic.[22] Other authors have demonstrated that dorsal angulation is associated with decreased functional results.[19, 20] Changes in sagittal tilt alter the biomechanics across the wrist joint. Pressure is dispersed over the entire articular surface of the distal radius and ulna at 11° of palmar tilt. As the sagittal tilt increases from 10° palmar tilt to 45° dorsal tilt, the axial load through the ulna increases from 21-67% of the total axial force. At 40° of dorsal tilt, most of the axial load is borne by the dorsal aspect of the radioscaphoid and ulnocarpal articulations without any load on the radiolunate joint.[31]
Intra-articular step-off and gap on the distal radius articular surface has been studied extensively. It generally is agreed that more than 2 mm of articular displacement can lead to radiographic osteoarthritis (OA).[32, 33, 34, 35, 36] However, radiographic OA does not always indicate poor functional outcome.[19, 22, 32, 37, 38, 39]
Trumble et al adds that intra-articular fractures with more than 1 mm displacement should be treated aggressively because, for example, the wrist articular cartilage is not as thick as that of the knee .[18] This decreased thickness of cartilage may consequently diminish the ability of the wrist to remodel residual articular incongruity.[40, 36] Anatomic reduction of the articular surface is recommended to decrease radiographic OA and optimize functional outcome.[29, 32, 34, 37]
In elderly patients, poor radiographic results do not necessarily signify poor functional outcomes.[22, 38] Satisfactory functional outcomes, regardless of radiographic results, are observed in patients older than 60 years—not because they are older, but because of their lower functional demands. Therefore, nonoperative treatment of distal radius fractures can yield satisfactory outcomes, especially in patients with low functional demands and in patients who are poor operative candidates.[2, 38]
See Pathophysiology.
There are no contraindications to the nonsurgical management of a closed distal radius fracture. Indications for surgical treatment should be based on radiographic findings after initial reduction, expected functional needs, associated medical conditions, and other traumatic injuries.
Open fractures also necessitate emergent surgical intervention and should be treated according to accepted principles. The decision for initial versus delayed placement of hardware should be based on the level of wound contamination and on the ability to achieve soft-tissue coverage over the implants
Workup
Copyright © www.orthopaedics.win Bone Health All Rights Reserved