

Low bone mass is extremely common among patients awaiting solid organ transplantation. A large and rapid decrease in bone mineral density (BMD) occurs within the first year following virtually all forms of solid organ transplantation. This decrease in BMD is associated with increased fractures (see the image below).[1, 2, 3] In a large series of abdominal organ and heart transplants from Northwestern University, Ramsey-Goldman et al reported a fracture incidence 5-34 times higher than in historical controls.[4]
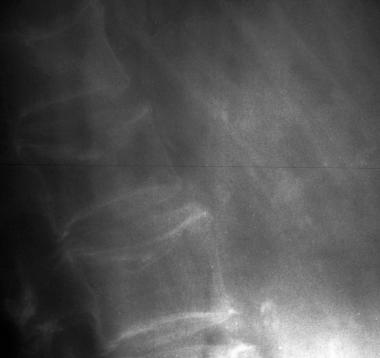 Osteoporotic spine. Note the considerable reduction in overall bone density and the lateral wedge fracture of L2.
Osteoporotic spine. Note the considerable reduction in overall bone density and the lateral wedge fracture of L2.
Traditionally recognized risk factors for osteoporosis include white race, low body weight, estrogen or androgen deficiency, calcium and/or vitamin D deficiency, and thyroid hormone excess. In addition to these traditional risk factors, other risk factors are associated with solid organ transplantation.
Even before transplantation, bone homeostasis may be adversely influenced by the disease process or diseased organ itself (eg, liver, lung, or kidney failure). Moreover, patients are often exposed to therapeutic agents such as steroids, heparin, or loop diuretics, which promote negative calcium balance and bone loss. After transplantation, high-dose steroid therapy and immunosuppression further promote bone loss and fracture development. To quote Elizabeth Shane, a recognized leader in this field, "Immunosuppression insults an already compromised skeleton."
Long-term survival following organ transplantation has improved considerably. Because patients often wait 2 or more years before transplantation, this represents an opportunity to prevent further bone loss and to help restore what may already have been lost. The clinical focus should be to both optimize bone mass before transplantation and to prevent bone loss in the postoperative period.[5]
For other discussions on osteoporosis, see the topics Osteoporosis and Pediatric Osteoporosis, as well as Bone Markers in Osteoporosis and Nonoperative Treatment of Osteoporotic Compression Fractures.
NextOsteoporosis is very common among patients awaiting lung transplantation. Shane and colleagues studied 70 patients awaiting transplant for end-stage lung disease and found osteoporosis in 30% at the lumbosacral (LS) spine and in 49% at the femur neck. Osteopenia (low bone mass) was noted at those sites in 35% and 31% of patients, respectively.[6] In other words, only a minority of patients awaiting transplant had normal bone density. Ferrari et al also prospectively evaluated changes in bone mass in 21 consecutive lung transplant candidates and confirmed this increased osteoporosis prevalence.[7] Prior to transplantation, BMD was decreased at all sites measured, and 35% of patients awaiting transplant had established osteoporosis, as defined by the World Health Organization (WHO).
Aris et al reported that nearly half (45%) of patients with end-stage lung disease awaiting transplant were at or below the fracture threshold. Moreover, following lung transplantation, nearly three quarters (73%) of patients were at or below the fracture threshold.[8] The prevalence rate of documented osteoporotic fractures was found to be 29% in patients with emphysema and 25% in patients with cystic fibrosis. Not surprisingly, the posttransplant BMD t- score was predicted by cumulative steroid dose.
Patients awaiting lung transplantation are at increased risk for osteoporosis because of the following:
Cystic fibrosis, a common indication for transplantation, is itself associated with low bone mass and fragility fractures because of (1) delayed puberty and hypogonadism and (2) chronic malnutrition with pancreatic insufficiency causing calcium and vitamin D malabsorption. Despite the common practice of giving supplemental oral vitamin D in patients with cystic fibrosis, the usual daily doses of 400-800 IU of vitamin D are often ineffective in maintaining normal vitamin D stores. Donovan et al found that 40% of patients with cystic fibrosis receiving that dosage of vitamin D were frankly vitamin D deficient.[9]
In Shane and colleagues' 1996 series, vitamin D deficiency was noted in 36% of patients with cystic fibrosis awaiting transplantation, although vitamin D deficiency was also very common among other patients with end-stage lung disease.[6] In this same series, 20% of patients with chronic obstructive pulmonary disease awaiting transplant had vitamin D deficiency, which was associated with more severe demineralization at the LS spine and hip.
Significant glucocorticoid exposure is nearly universal in persons with end-stage lung disease. Israel et al reported that even inhaled corticosteroids lead to a dose-related decline in bone density at the hip.[10] Similarly, van Staa et al reported that vertebral, nonvertebral, and hip fractures occur with increased frequency in association with inhaled corticosteroids.[11] Note that only very few patients receiving long-term glucocorticoid therapy in Shane and colleagues' 1996 study were simultaneously receiving an effective antiresorptive agent for osteoporosis prevention.[6, 12]
As with patients awaiting lung transplantation, only a minority of patients awaiting cardiac transplantation have normal bone density. Shane et al studied 101 patients with advanced congestive heart failure who were awaiting transplantation. Only 50% and 47% had normal BMD at the LS spine and total hip, respectively.[13]
The reasons for this are likely multifactorial. Patients with end-stage congestive heart failure are uniformly exposed to potent loop diuretics that promote negative calcium balance, and they often have coexisting renal disease and hepatic congestion from their low-flow state. Low serum concentrations of 25(OH) vitamin D and 1,25-dihydroxyvitamin D with secondary hyperparathyroidism are quite common. As the disease advances, patients become less mobile and have less sun exposure.
Not surprisingly, Shane et al found that vitamin D deficiency was significantly more common in the patients with more severe heart failure.[13] However, Iqbal et al did not find significantly low BMD in the lumbar spine and hip, relative to age and sex, in ambulatory patients with heart failure who were waiting for cardiac transplantation.[14] )
Vertebral fractures are highly prevalent among cardiac transplant recipients, with a fracture prevalence of 18-50% reported across various series.[15] Among 47 patients monitored by Shane and colleagues postcardiac transplant, 17 sustained 34 fractures after 1 year, despite having adequate calcium and vitamin D. At least 1 fracture was experienced by 54% of the women and 29% of the men. The vast majority (85%) of fractures occurred in the initial 6 months following transplantation, with most fractures involving the spine. Women with low femur-neck density were significantly more likely to sustain posttransplantation fractures.[16]
Following cardiac transplantation, LS spine bone density typically declines 6-10% in the first 6 months, after which it stabilizes. Hip density similarly declines in the first year, dropping 10-15% below pretransplant levels.
After the first year, bone loss usually slows, and LS spine density may actually increase slightly in the third year.[15] In comparison, after the second and third years following cardiac transplant, the one-third distal radius, a site enriched for cortical bone and susceptible to parathyroid hormone (PTH) action, shows evidence of continued bone loss.[15]
Since most bone mass is accrued by the late teenage years, concern exists whether patients who undergo transplant during these critical years would fail to accrue normal bone mass or delay achievement of peak bone mass. A cross-sectional case control study of 9 patients who were 12-16 years old at the time of cardiac transplantation found that at 8-16 years posttransplant, transplant recipients had shorter stature than calculated midparental height would predict.[17] Biochemical parameters suggested renal impairment with secondary hyperparathyroidism, without a difference in vitamin D levels between the treatment and control groups.
The authors suggest a pathogenic role for PTH in the osteoporosis in this population. Most of the patients received glucocorticoids at the time of study, yet glucocorticoids are usually associated with low bone turnover and suppressed bone formation. High bone turnover was noted in the study with markedly lower BMD at the forearm. Whether the increased bone turnover observed would ultimately be associated with ongoing loss of bone or continued bone growth was not clear from this cross-sectional study.[17]
In a noncontrolled report, the incorporation of mycophenolate mofetil in the immunosuppressant regimen has successfully reduced steroid requirements in a subset of patients with symptomatic osteoporosis after cardiac transplantation. However, in the small number (12) of patients studied, this did not result in an improvement of BMD after 1 year.[18]
A minority of patients awaiting liver transplantation have normal bone density. In a large series of 243 consecutive patients undergoing evaluation for liver transplantation, only 15% had normal bone density.[19] Moreover, vertebral fractures were present in 35% of patients prior to transplantation in this same population.[20]
End-stage liver disease (ESLD) itself is associated with osteoporosis. Vitamin D deficiency is extremely common among patients with cirrhosis who are awaiting transplant. Cirrhosis is associated with significantly depressed levels of 25-hydroxyvitamin D-3, 1,25-dihydroxyvitamin D, osteocalcin, and PTH.[21] Cirrhosis is also associated with low osteocalcin levels and with histomorphometric evidence of decreased bone formation.
In chronic liver disease, low vitamin D levels predict bone loss.[22] In one study of 27 patients awaiting orthotopic liver transplantation, 74% had subnormal 25-hydroxyvitamin D levels at baseline. Vitamin D levels were inversely associated with more advanced Child-Pugh classification, and more advanced Child-Pugh class is associated with increased bone loss at the LS spine.[23]
Chronic obstructive liver disease may interfere with the enterohepatic circulation of vitamin D metabolites. The cholestasis observed in persons with primary biliary cirrhosis (PBC) may inhibit normal osteoblast function by an uncertain mechanism, resulting in a low bone turnover osteoporosis. Although the specific pathophysiological mechanisms in PBC have not been defined, histomorphometry findings reveal depressed bone formation and inactive remodeling.[24, 25]
Polymorphism in the gene encoding collagen type I alpha1 (COLA1) Sp1 is a recently recognized genetic predictor of peak bone mass. Not surprisingly, COLA1 has been found to be a marker of bone mass in patients with PBC, although the degree of cholestasis remains the more important risk factor for osteoporosis.[26]
Along the same line, allelic polymorphism of the vitamin D receptor is also thought to be predictive of BMD in healthy patients and in patients with primary osteoporosis. The vitamin D receptor genotype influences bone loss after liver transplantation and also predicts lower BMD in patients with PBC. Specifically, the bb genotype is partially protective against posttransplant bone loss.[27, 28]
In general, more severe liver disease and more severe cholestasis are associated with more severe bone loss.[29] In fact, bone loss was predicted by the degree of hyperbilirubinemia in a Swedish study.[23] Cholestasis-related osteopenia appears to be more severe than osteopenia associated with viral liver disease. In one cross-sectional study, BMD z scores were more than twice as depressed in cholestatic patients than in patients with viral liver disease, although viral liver disease is itself associated with significant osteopenia.[30]
In a study of 32 consecutive viral cirrhotic patients in whom alcoholism had been excluded, Gallego-Rojo et al found reduced BMD at all sites measured, and established osteoporosis in 53% of patients. Serum immunoglobulin F-1 levels were lower in viral cirrhotic subjects than in control subjects, and levels differed significantly between cirrhotic patients with and without osteoporosis. Therefore, low immunoglobulin F-1 levels may play a role in osteopenia associated with viral cirrhosis.[30, 31]
Alcohol abuse is a well-known cause of cirrhosis; it is also a well-known risk factor for osteoporosis, which is likely multifactorial in origin. Malnutrition and chronic pancreatitis are common in persons with alcoholism and are frequently associated with concomitant vitamin D and magnesium depletion.[32, 33] In humans, magnesium deficiency is known to result in hypocalcemia, impaired PTH secretion, and low serum concentrations of 1,25-dihydroxyvitamin D.[34] Ultimately, alcohol itself may directly suppress bone formation, as evidenced by a direct correlation between bone GLA protein levels and days of abstinence from alcohol.[35]
Hypogonadism is a known risk factor for osteopenia and occurs frequently in persons with alcoholism and ESLD. Both low testosterone and high sex hormone–binding globulin levels correlate with worsening Child-Pugh classification.
Monegal et al have documented osteoporosis in 43% of cirrhotic patients at the time of referral for liver transplantation. In the first year post transplantation, bone mass declined further, with LS spine BMD falling 3.5-24%.[21, 36] By 3 years after liver transplantation, a third of the patients developed fractures. In this cohort, age and low bone mass were identified as pretransplant risk factors for fracture.
As with cardiac transplantation, the highest fracture incidence rate occurs in the first year following liver transplantation. Estimates range from 24-65%, with the highest rate being reported in women with PBC. In a study of women with PBC by Eastell et al, BMD at the LS spine was inversely related to the severity of liver disease. The overall rate of bone loss in half the patients with PBC was twice that of healthy controls. Following liver transplantation, BMD in the LS spine fell at 3 months and was associated with atraumatic fractures in 13 of 20 women.[29]
Histomorphometric analysis of transiliac bone biopsy specimens prior to and 3 months after liver transplantation in 21 patients with chronic liver disease demonstrated a highly significant and quantitatively large increase in bone turnover within the first 3 months after liver transplantation. The bone turnover rate was low preoperatively, with thinner walls and erosion depth. The bone formation rate increased after transplantation. A small increase in osteoid seam width was noted postoperatively, with a decrease in the mineralization lag time.[37]
A high incidence of vertebral fracture in the first 3 months after liver transplantation is well recognized. As in other cohorts, prevalent vertebral fracture pretransplant is an important risk factor for the subsequent development of fracture in the liver transplant population.[20]
Significant recovery of BMD following transplantation was noted in this population. By 12 months, the median BMD at the LS spine was similar to the pretransplantation BMD; by 24 months, BMD was actually 5% higher than the pretransplant baseline. Bone mass may return to normal within 2-3 years following liver transplant.[29]
Several other studies have reported some long-term recovery of BMD in the liver transplant population. In a Dutch cohort treated with a prednisolone- and azathioprine-based immunosuppressant regimen and with follow-up care extending to 5-15 years, improvement in BMD was mainly observed in the second postoperative year, with stabilization thereafter.[38] Despite this interval improvement, approximately one third of patients were left with a BMD below the fracture threshold. In general, the outcome was less favorable in men than in women and in patients who received transplants for cholestatic liver disease, who may initially have had more severe bone disease.[38]
Not all series have demonstrated recovery and stabilization of BMD following liver transplantation, and some show continued decline.[30]
The most common sites to fracture in the liver transplant recipient are the vertebrae and ribs. In a prospective study by Ninkovic et al in 37 patients with ESLD, vertebral fractures were evident in 35% of patients before transplantation, and new fractures developed by 3 months after transplantation in 27%. Although osteoporosis (defined as a t score <-2.5) was found in 39% of patients prior to transplantation, BMD did not reliably predict fracture risk. However, subsequent vertebral fractures were significantly more common in those with a prevalent vertebral fracture.
Nearly all cross-sectional studies of renal transplant recipients demonstrate that BMD is below normal levels. A fracture prevalence of 5-11% has previously been reported in cross-sectional studies, which is similar to or greater than rates observed in women with postmenopausal osteoporosis.[39, 40] Hyperparathyroidism preceding renal transplantation accelerates trabecular bone loss at the spine in the early transplant period.[41]
Most patients with end-stage renal disease (ESRD) are hypogonadal and have some degree of renal osteodystrophy. (For a comprehensive discussion of renal osteodystrophy, which is beyond the scope of this chapter, please see Goodman et al, 2003.[42] ) Most patients with ESRD have been exposed to drugs that negatively affect bone metabolism. In one large series of 250 such patients, risk factors for low bone mass included secondary amenorrhea, prior failed renal transplantation, chronic metabolic acidosis, and chronic heparin and aluminum exposure. Having had a prior failed renal transplant is probably associated with increased immunosuppressant exposure to prevent rejection and to hyperphosphatemia associated with osteomalacia and osteoporosis.[43]
In recent years, continuous use of aluminum-containing phosphate binders has largely been abandoned; therefore, aluminum toxicity is not presently a significant problem in transplant-related bone disease.[44, 45] However, adynamic bone disease has become increasingly prevalent in the chronic kidney disease population, evident in 27% of transiliac bone biopsy specimens[46] and up to 50% of renal transplant patients prior to bisphosphonate treatment for osteoporosis.[47]
After successful renal transplantation, secondary hyperparathyroidism usually resolves gradually, with normalized vitamin D metabolism and creatinine clearance (CrCl). PTH levels frequently normalize or improve by 1 month posttransplant because of immediate improvement in phosphate retention. Cortical bone density improves secondarily, with significantly better z scores at the distal radius occurring by 6 months after renal transplantation. However, in approximately a third of cases, persistent hyperparathyroidism and hypercalcemia are noted.[48]
In cases of refractory hyperparathyroidism in which surgery is indicated, parathyroidectomy has been associated with a marked improvement in BMD.
With successful renal transplantation, improvement also occurs in aluminum bone disease and dialysis-associated amyloidosis.[49]
Overall, the transplant-related bone disease in kidney recipients does not appear to be as severe as in other solid organ transplant recipients, with the possible exception of kidney recipients with type 1 diabetes.[50] This is perhaps because kidney transplant recipients are younger on average than other organ recipients at the time of transplantation and they may have had better recognition and management of pretransplant bone disease. Kidney transplant recipients may receive lower doses of immunosuppression overall. Rejection may also be more easily detected in renal recipients and, therefore, treated earlier than in other solid organ transplant recipients, resulting in lower total doses of immunosuppression.
Simultaneous pancreas-kidney transplantation (SPKT) successfully restores euglycemia and insulin independence while reversing uremia in patients with ESRD due to diabetic nephropathy. While diabetic patients constitute approximately 20% of those receiving renal transplants, virtually all patients receiving both a pancreatic and renal transplant have type 1 diabetes mellitus. Because type 1 diabetes mellitus itself predisposes to cortical osteopenia and low bone turnover, patients with the condition are clearly at greater risk of transplant-associated bone disease and fracture.[50] The reasons for this are likely multifactorial.
Most patients with type 1 diabetes mellitus have not yet achieved peak bone mass before the onset of diabetes, and long-standing insulin deficiency may compromise bone mass. Multiple studies have documented that patients with type 1 diabetes have reduced BMD at all sites measured, with a high prevalence of osteoporosis. Munoz-Torres et al examined a cohort of 94 consecutive Spanish patients (aged 20-56 y) with type 1 diabetes of 1-35 years' duration presenting to a diabetes clinic for therapy and observed reduced BMD at all sites; 19.1% of patients had osteoporosis.[51]
Poor glycemic control, the presence of diabetic complications, and smoking are also associated with a lower BMD in multiple studies. In a study by Campos-Pastor, diabetic patients with retinopathy, for example, were much more likely to exhibit osteopenia or osteoporosis.[52] However, the presence of retinopathy and the degree of glycemic control are clearly not independent variables because the mean glycosylated hemoglobin value was significantly higher in the group with retinopathy (8.5% vs 7.1%). BMD also correlates with body mass index, which is dependent upon the degree of insulinization and glycemic control.
Campos-Pastor et al observed 62 patients with type 1 diabetes and found that after 7 years of intensive insulin therapy, BMD stabilized at all sites. A significant fall in tartrate-resistant alkaline phosphatase levels (4.3 vs 2.7 IU/L) and a significant rise in intact PTH levels (28 vs 40 ng/L) were also observed.[52]
Addesso et al and Shane et al found that in a type 1 diabetic cohort awaiting SPKT, osteoporosis and osteopenia, respectively, were present in 11% and 21% of patients at the LS spine, 29% and 54% at the femur neck, 25% and 61% at the Ward triangle, and 14% and 64% at the trochanter.[13] Of the 20 patients with available fracture data, 15 (75%) had already sustained a fracture while awaiting transplantation. In total, 12 extremity fractures and 3 rib fractures occurred. The mean age of the patients was 39 years, with a mean duration of type 1 diabetes mellitus of 23 years.
In a Swedish cohort of renal transplant recipients followed by Nisbeth and colleagues, a symptomatic osteoporotic fracture occurred in 40% of the patients with type 1 diabetes but in only 11% of renal transplant recipients without diabetes.[50] Symptomatic bone disease was examined in this cross-sectional study using questionnaires and hospital records in 193 renal transplant recipients with functional grafts 6 months to 23 years after transplantation. Most fractures occurred within the first 3 years posttransplant. Fractures in diabetic patients were often multiple and were located mostly in the appendicular skeleton, ankles, and feet.
A cross-sectional study by Smets et al in 31 Dutch patients at least 12 months following successful SPKT (mean 40 ± 23 mo) found a high prevalence of secondary hyperparathyroidism, increased bone turnover, and fractures.[53] All patients were insulin independent with a mean CrCl level of 64 ± 21 mL/min. Secondary hyperparathyroidism was noted in 55% of patients. Increased osteocalcin, a marker of increased bone turnover, was present in 45%. However, accurate determination of osteocalcin in the SPKT patient is complex, because the clearance of osteocalcin is reduced by a decreased CrCl and osteocalcin levels are raised by hyperglycemia.
In the Smets et al study, osteoporosis (t score <-2.5 standard deviation [SD]) was found in 23% of patients at the lumbar spine, a predominantly trabecular site, but in 58% of patients at the femur neck, a predominantly cortical site. Fractures, which were primarily nonvertebral, were found in 45% of the SPKT patients studied.[53]
Smets and colleagues subsequently prospectively followed 19 consecutive SPKT patients who began taking the synthetic vitamin D analog alfacalcidol (0.25 mcg/d) at the end of the first posttransplantation year, and found a small but significant increase in LS spine BMD in the first 6 months (+1.7 ±2.1%).[53] These patients had, on average, approximately 25 years of type 1 diabetes. None of the patients had osteoporosis at the lumbar spine prior to transplantation, although 7 (37%) had cortical osteoporosis pretransplant.
The authors note that the cumulative prednisone dose was similar in most patients. A significant increase in serum calcium (+0.08 ± 0.02 mmol/L) was noted in the 6 months after alfacalcidol was begun, which was not associated with a significant decrease in PTH, so perhaps the dose was not sufficient to fully normalize PTH secretion.[53]
This paper confirmed a pattern of heightened trabecular and cortical bone loss during the first 6 months after SPKT. In contrast to other studies,[49] however, which suggested continued bone loss up to 18 months postrenal transplant, this study in SPKT recipients suggested stabilization of BMD after the first 6 months posttransplant.
Compared with their baseline, 9 patients (47%) had osteoporosis at the femoral neck, and only 1 patient (5%) developed osteoporosis at the lumbar spine. In this cohort, all fractures occurred more than 1 year posttransplantation; about one third of patients experienced a fracture over a mean follow up period of approximately 3.3 years.[54]
In conclusion, low bone mass is highly prevalent both prior to and following successful SPKT and is associated with a high fracture prevalence. Cortical bone loss is unusually prevalent in this specific transplant population, possibly due to both cortical osteopenia and persistent hyperparathyroidism.
Routinely administered posttransplant immunosuppressants play a central role in the pathogenesis of bone loss and fracture. Regimens typically include glucocorticoids (at high dose initially), cyclosporin A (CsA), tacrolimus FK506, azathioprine, or mycophenolate mofetil. Because they are always administered simultaneously, sorting out the independent effects of immunosuppressants from those of glucocorticoids is difficult, if not impossible. Immunosuppressant doses are typically higher in liver and heart transplantations than in renal transplantation, contributing to the more advanced osteopenia seen in those groups.
While a detailed discussion on glucocorticoid-induced bone loss is beyond the scope of this article, glucocorticoids are known to induce osteoporosis. An increased risk of vertebral fracture has been associated with an oral dose of prednisolone of as low as 2.5 mg/d, which is approximately equipotent to prednisone at 2.5 mg/d. Glucocorticoids are commonly prescribed in high doses, up to 120 mg of prednisone or its equivalent daily during periods of acute rejection and immediately posttransplantation.
Glucocorticoids promote bone loss through a variety of simultaneously operating mechanisms, as follows:
Glucocorticoids result in a disproportionate loss of cancellous or trabecular bone, possibly because trabecular bone has an inherently greater rate of turnover than cortical bone. Serum bone GLA protein, osteocalcin, is also inhibited. Thus, glucocorticoids induce a low-turnover osteopenia and disproportionately affect trabecular bone.[49]
With the advent of the cyclosporines in the early 1980s, graft survival markedly improved owing to decreased organ rejection. The introduction of cyclosporines allowed steroid doses to be substantially reduced. At the time, the hope was that the harmful effects of immunosuppression on the skeleton would be ameliorated. Unfortunately, this was not the case. The lowest effective dose of glucocorticoids is recommended to minimize loss of bone mass and risk of osteonecrosis (a common complication in the first 2 years following transplant).
Like glucocorticoids, CsA causes severe and rapid trabecular bone loss. However, unlike glucocorticoids, CsA results in accelerated bone turnover, with both increased formation and resorption. The bone histomorphology resembles that of the oophorectomized female rat. Antiresorptive agents, such as estrogen, alendronate, and calcitonin, can largely prevent this bone loss. Alendronate specifically prevents CsA-induced osteopenia in rats, maintaining trabecular bone volume at the tibia.
Some investigators have speculated that this effect of cyclosporine may be mediated through testosterone because cyclosporine suppresses the hypothalamic-pituitary-gonadal axis and lowers serum testosterone levels in rats and in human transplant patients. Some evidence suggests that cyclosporine may have a direct testicular effect. Examination of rat testes after CsA exposure has revealed decreased LH-receptor numbers and dramatically decreased serum and intratesticular testosterone. Altered testicular cytochrome P-450 activity is reported due to suppressed heme formation and the steroidogenic activities that rely on it, such as 17-hydroxylase and side-chain cleavage enzymes.
Others have speculated that CsA may have a direct pituitary or hypothalamic effect, inducing hypogonadotrophic hypogonadism and a blunted response of LH/follicle-stimulating hormone (FSH) to gonadotropin-releasing hormone. While CsA may have both central and direct testicular effects, CsA-induced bone loss is not prevented by testosterone administration in the rat model and did not correlate with bone turnover or histomorphometry findings.
Tacrolimus is a fungal macrolide that is less nephrotoxic and more potently immunosuppressive than CsA. In rats, this has been reported to cause high-turnover bone loss of even greater magnitude than that caused by CsA.[55, 56] Because tacrolimus is a more potent immunosuppressant, steroid doses may be reduced further with tacrolimus than with CsA.
A study in liver transplant recipients by Smallwood et al showed a nonsignificant tendency toward fewer patients with low bone density in the group receiving tacrolimus (n=112) compared with those receiving cyclosporine (n=25). Among the patients weaned from prednisone, the patients treated with tacrolimus were less likely to have low BMD (36.2% vs 68.8%).[57] Overall, rates of bone loss have been similar in heart, liver, and kidney transplant recipients receiving either tacrolimus or CsA.
Rapamycin inhibits downstream signaling from the mammalian target of rapamycin (mTOR) proteins, a signaling pathway promoting tumor growth. Rapamycin binds to the FK506 binding protein, and this complex then binds to mTOR and prevents interaction of mTOR with target proteins in the signaling pathway. Short-term administration of rapamycin causes no trabecular bone loss and potentially has bone-sparing effects.[58]
Two open-label, randomized, phase-2 studies comparing sirolimus versus cyclosporine examined bone metabolism with markers of bone turnover, osteocalcin, and urinary N-telopeptides measured over a 1-year period in 115 patients receiving CsA or sirolimus with azathioprine and glucocorticoids or mycophenolate with glucocorticoids. In the patients treated with CsA, serum osteocalcin and urine N-telopeptides were consistently higher compared with those receiving sirolimus.[59]
Osteoprotegerin (OPG) is an antiresorptive cytokine and a potential mechanism for immunosuppressant osteopenia. A member of the tumor necrosis factor–receptor superfamily, OPG is a critical regulator of bone resorption. OPG inhibits terminal differentiation and activation of osteoclasts.[60] OPG deficiency causes osteoporosis in mice, and, when administered to ovariectomized rats, OPG decreases osteoclast activity and restores normal bone mass.
OPG is produced by osteoblasts and arterial cells, and inhibits osteoclast function by neutralizing receptor activator of NF-kappa B ligand (RANKL).
Injections of OPG are well tolerated and rapidly decrease markers of bone resorption, urine N -telopeptide, and bone alkaline phosphatase. CsA, rapamycin (sirolimus), and tacrolimus (FK506) significantly decrease OPG mRNA and protein levels in undifferentiated marrow stroma (44-68%). A reciprocal, significant increase in RANKL mRNA levels (60-120%) is also seen with these agents.
In contrast, the potential bone-sparing effect of rapamycin may be explained by the increase in OPG-mRNA seen in mature osteoblasts.[61] Renal transplant recipients treated with intravenous zoledronate (a third-generation bisphosphonate) demonstrated significant elevations of OPG over the first 6 months of treatment, consistent with osteoclast inhibition.
Kidney transplant patients generally experience less transplant-related bone disease than cardiac[12] (50% vertebral fracture prevalence), lung, or liver transplant patients. This may be because renal transplant recipients are younger (on average) at the time of transplantation and may have better management and recognition of metabolic bone disease prior to transplantation; moreover, they may receive lower doses of immunosuppression overall.
Kidney transplant patients with type 1 diabetes mellitus and SPKT recipients are probably an exception to this general statement because, for the reasons outlined above, they are predisposed by their type 1 diabetes to cortical osteopenia.
Women are at greater risk for all forms of osteoporosis. Women with small frames and low body weight (ie, < 70 kg) are particularly at risk; white or Asian women are at highest risk. Postmenopausal women (particularly women with early spontaneous or surgical menopause) are at increased risk because of estrogen deficiency.[62] Women with a history of prolonged periods of amenorrhea and hypogonadism are also at increased risk
The pretransplant bone evaluation should include a careful history with particular attention to risk factors for osteoporosis. Any personal history of fracture is particularly relevant because prior fracture predicts future fracture.[55] Any family history of osteoporosis or fragility fractures is also relevant. A history of loss of height suggests established osteoporosis and occult thoracic compression fracture.
The review of systems should include a review of gonadal function because a history of amenorrhea, decreased libido, or erectile or orgasmic dysfunction could suggest underlying hypogonadism, predisposing to osteopenia.
The review of systems should specifically inquire about bone pain, myalgias, or myopathic symptoms, which could suggest occult vitamin D deficiency or osteomalacia.
Significant constitutional symptoms or symptomatic anemia could suggest occult multiple myeloma as a cause of osteopenia, in the appropriate clinical context.
Because immobility is known to promote negative bone balance, any history of periods of prolonged bed rest is also relevant.
A careful medication history should be taken for past anticonvulsant use because these medications can derange vitamin D metabolism. Heparin, loop diuretics, and steroids (both oral and inhaled) are associated with negative calcium balance. Any history of tobacco or alcohol abuse is relevant because the use of these substances is an established risk factor for osteoporosis.
A dietary history should be obtained. This should include an estimate of daily calcium intake. An overview of lifelong dairy intake or avoidance, lactose intolerance, malabsorption, or celiac disease,[56] which predispose the patient to vitamin D deficiency, is also relevant. A history of sun avoidance is similarly important. Overall nutritional status, including any prior periods of malnutrition or energy (caloric) restriction (eg, anorexia nervosa) should be assessed.
A history of dietary supplement intake is also relevant. Observational data suggest that excess vitamin A intake (eg, as retinol) is associated with an increased hip fracture risk.[63]
Record height and weight, along with any loss of height or kyphosis, which could suggest occult compression fracture.
The examiner should be alert for the presence of blue sclerae, which suggest underlying osteogenesis imperfecta.
Any scoliosis or other regions of bony deformity should be noted. These deformities have the potential to render bone density determinations inaccurate in these regions, and the physician or technician performing bone densitometry should be made specifically aware of them.
Any areas of bone tenderness or pain, which could suggest occult fracture, avascular necrosis, or osteomalacia, should prompt further diagnostic evaluation.
Incapacitating pain or tenderness in the lower extremities or bones of the feet following organ transplantation could suggest the calcineurin-inhibitor–induced pain syndrome (CIPS), an unusual side effect of cyclosporine or tacrolimus.
CIPS may be accurately diagnosed by its typical presentation, negative inflammatory markers (erythrocyte sedimentation rate [ESR], C-reactive protein [CRP]), and characteristic magnetic resonance imaging finding with altered bone marrow signal. The reduction of cyclosporine or tacrolimus trough levels and the administration of calcium channel blockers has led to relief of pain, although in some cases bone marrow edema on follow-up MRI and pain syndrome resolved over 3 months without specific therapy.[64, 65]
Since even patients with entirely normal pretransplant BMD experience fractures following solid organ transplantation, it is not possible to reliably predict who will sustain a fracture. Thus, it has been recommended that all transplant candidates undergo an evaluation of bone health.[66] The following laboratory studies are indicated in the pretransplant evaluation of all patients awaiting solid organs[61, 67] :
The Kidney Disease Outcomes Quality Initiative (KDOQI) has published comprehensive Clinical Practice Guidelines for interventions for the prevention of bone disease following renal transplantation.[68]
During the first week after kidney transplantation, serum levels of phosphorus should be monitored daily due to high urinary excretion rates associated with improving renal function. While there is not an absolute consensus as to the level of hypophosphatemia necessitating replacement, kidney transplant recipients demonstrating persistently low serum phosphate levels (< 2.5 mg/dL [< 0.81 mmol/L]) should probably receive supplemental phosphate.[69]
Since phosphate supplementation may worsen hyperparathyroidism—with a decrease in serum calcium, increase in PTH, and decrease in 1,25-dihydroxyvitamin D—KDOQI guidelines suggest that calcitriol or vitamin D analog therapy may be necessary if phosphate supplementation is required.
In the first 3 months following renal transplant, levels of calcium and phosphate should be monitored at least weekly to achieve a desired phosphorus target range of 2.5-4.5 mg/dL (0.81-1.45 mmol/L). More than 3 months after transplant, K/DOQI guidelines state that PTH, calcium, and phosphorus are to be measured at a frequency clinically appropriate to the achieved glomerular filtration rate (GFR).
While PTH levels usually return nearly to normal by 1 year following renal transplant, hypercalcemia due to preexisting hyperparathyroidism is not rare after renal transplant. Persistent hyperparathyroidism has been associated with higher pretransplant PTH and calcium levels, in addition to a longer period of dialysis preceding transplantation.[70]
Hypercalcemia usually improves as hypertrophic parathyroid glands involute with improving GFR. Since hypercalcemia that requires parathyroidectomy may persist in up to 5% of renal transplant recipients, monitoring of calcium is prudent. The development of spontaneous fractures in a kidney transplant recipient in the presence of hyperparathyroidism is thought by many experts to be an additional indication for parathyroid resection (K/DOQI guidelines).
As in ESRD, metabolic acidosis may persist following renal transplant, inducing bone calcium loss and inhibiting osteoblast function.[71] In addition, systemic metabolic acidosis blunts the anabolic effects of insulin-like growth factor 1(IGF-1), the downstream mediator of growth hormone action,[72] and reduces synthesis of 1,25-dihydroxyvitamin D at the proximal renal tubules, thereby limiting dietary calcium absorption.[73] Correction of metabolic acidosis may be advisable since bone acts as a buffer for correction of acidosis, further compromising the at risk skeleton of the transplant recipient.
A 24-hour urine calcium study may also be considered in the absence of loop diuretic therapy because glucocorticoid administration can be associated with significant hypercalciuria, which is exacerbated by aggressive calcium and vitamin D therapy.[58] In this instance, thiazide diuretics could potentially be considered as a therapeutic strategy since they decrease urine calcium excretion and increase calcium absorption in glucocorticoid-treated patients and may attenuate hip fracture risk.[74, 75, 76]
A low 24-hour urine calcium value could suggest malabsorption or vitamin D deficiency, necessitating higher doses of vitamin D.
Biochemical markers of bone remodeling are not uniformly indicated in the pretransplant evaluation. Although they may help to assess the rates of bone remodeling, changes in their concentration are quite variable and not uniformly predictive of fracture risk.[77]
Because use of aluminum-containing binders in the ESRD population has declined considerably in recent years, a serum aluminum level is optional in patients awaiting renal transplantation.
Such testing should be reserved for dialysis patients in whom aluminum-related bone disease is a likely possibility (ie, patients with renal osteodystrophy and prior exposure to aluminum-containing binders or aluminum-containing dialysate).[78]
The predictive value of serum aluminum values for predicting aluminum-related bone disease has been questioned,[79] and aluminum staining at the mineralization front on bone biopsy specimens may more accurately reflect the histopathology found in patients on dialysis.[80]
In the appropriate clinical context of anemia or back pain or when a secondary cause of osteopenia is suggested, a serum protein electrophoresis study should be considered.
Bone densitometry measurements by dual energy x-ray absorptiometry (DEXA) scanning of the LS spine and hips should be performed on all patients prior to transplantation.
DEXA scanning should be performed not only to screen for preexisting bone loss in this medically ill population predisposed to osteoporosis but also to establish a baseline in patients who will require long-term administration of glucocorticoids as part of an immunosuppressive posttransplantation regimen.[81]
Guidelines established by the American College of Rheumatology and the UK Consensus Group recommend that patients receiving daily glucocorticoid at doses of 7.5 mg or more of prednisolone for 6 months or longer should have a BMD measurement.[82]
Bone density of the distal radius should also be measured in patients with renal osteodystrophy and any evidence of hyperparathyroidism.
The Kidney Disease Outcomes Quality Initiative has recommended a DEXA scan at time of renal transplantation by DEXA to assess for the presence of development of osteoporosis.[83] DEXA scans are recommended at time of transplantation and 1 and 2 years following transplantation. Parenteral bisphosphonate should be considered when BMD t -score is less than or equal to –2 standard deviation.
Because the prevalence of occult, asymptomatic fractures (in particular, spinal compression fractures) is considerable, complete spine radiographs may be obtained (see images below).[61] Further, radiographs of any painful bone sites should be obtained to help exclude the presence of Looser zones or milkman fractures, which are pathognomonic for osteomalacia.
 Osteoporotic spine. Note the considerable reduction in overall bone density and the lateral wedge fracture of L2.
Osteoporotic spine. Note the considerable reduction in overall bone density and the lateral wedge fracture of L2.
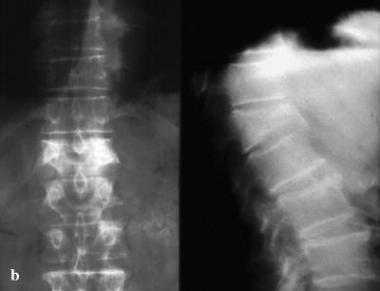 Anteroposterior and lateral radiographs of an L1 osteoporotic wedge compression fracture.
Anteroposterior and lateral radiographs of an L1 osteoporotic wedge compression fracture.
Because chronic pain and immobilization from fractures can significantly diminish quality of life, it has been recommended that patients with extremely low bone mass or osteoporotic fractures documented prior to transplantation be counseled about the increased fracture risk that follows transplantation.[81] Since even an entirely normal bone density pretransplantation is not protective against posttransplantation fracture, prophylaxis against bone loss should be given in all transplant recipients, without regard to baseline bone density.[66]
Any patient who meets World Health Organization (WHO) criteria for low bone mass (osteoporosis) should receive pharmacologic treatment similar to any other patient with osteoporosis or osteopenia. There are no specific therapies for posttransplantation osteoporosis approved by the US Food and Drug Administration. Therapeutic strategies are extrapolated from nontransplant situations and based on relatively small numbers of patients in clinical trials. Vitamin D and calcium alone are clearly insufficient to prevent transplant-related bone loss.
The aim of medical therapy should be to prevent bone loss and (if possible) to restore bone lost before transplantation. Guidelines established by the American College of Rheumatology and the UK Consensus Group[82, 84] recommend that patients who receive daily glucocorticoid at doses of 7.5 mg of prednisolone or more for 6 months or longer should begin preventive therapy. Transplant recipients clearly meet this criterion.
Postrenal transplant bone disease reflects the complexity of preexisting renal osteodystrophy, although many aspects of renal osteodystrophy improve with transplantation. Hyperparathyroidism may persist in a subset of patients.[85] Advise all patients to maintain an adequate total elemental calcium intake (ie, 1000-1500 mg) and to take supplements as necessary.
The dose of supplemental calcium should be individualized on the basis of dietary calcium intake, menopausal status, and underlying medical issues. For example, a pharmacologic dose of calcium administered to a renal transplant recipient with persistent secondary hyperparathyroidism could worsen hypercalciuria because of excess PTH action and could be contraindicated.
Administer vitamin D at 400-1000 IU/d to all patients. However, patients with malabsorption, cystic fibrosis, or PBC have higher vitamin D requirements,[9, 86] and 25-hydroxyvitamin D levels should be monitored to assess the adequacy of replacement.
Parent vitamin D at doses of 400-1000 IU daily is insufficient to prevent posttransplant osteoporosis. Active metabolites of vitamin D are more promising in this regard and may work by improving intestinal calcium absorption and directly or indirectly suppressing PTH secretion.[87]
In an uncontrolled 12-month study in 77 renal transplant recipients, Talalaj et al found that BMD remained stable at the LS spine (–0.2%) or modestly increased at the femur neck (1.3%) in subjects who received calcidiol 40 mcg with calcium 3 g daily. A significant decrease in vertebral deformities was notable in the calcidiol group.[88] In the untreated group, BMD significantly decreased, by 7.1% at the LS spine and 5.5% at the femur neck.
Garcia-Delgado et al reported that randomization immediately following cardiac transplant to 32,000 IU per week of oral calcidiol for 18 months increased LS spine BMD, whereas etidronate or nasal calcitonin was associated with a decrease in LS spine BMD.[89]
1-Hydroxylated vitamin D may be of particular use in clinical situations associated with accelerated bone loss. There is evidence that alfacalcidol increases trabecular BMD and prevents vertebral fracture. In newly postmenopausal women, alfacalcidol at 1 mcg/d prevented the accelerated loss of vertebral BMD over 3 years following the onset of menopause.
In a nontransplant population of patients with established glucocorticoid-induced osteoporosis, Ringe et al reported an impressive 52% relative risk reduction in new fractures over 3 years with alfacalcidol versus parent vitamin D-3.[90] Matched pairs were randomized to 1 mcg alfacalcidol with 500 mg calcium per day (n=103) or 1000 IU of vitamin D-3 with 500 mg calcium per day (n=101). The median percentage increase in spine BMD in the alfacalcidol group (+2.4%) was 4-fold greater than the parent vitamin D group (-0.8%) over 3 years. In addition, alfacalcidol decreased back pain to a greater degree than plain vitamin D-3.
Generally, side effects in both groups were mild, and only 3 patients in the alfacalcidol group and 2 in the vitamin D group had moderate hypercalcemia. Alfacalcidol would appear to be superior to plain vitamin D-3 in the treatment of established glucocorticoid-induced osteoporosis. At 0.5-1 mcg daily, others have found it useful in preservation of spinal bone density in rheumatoid arthritis as well as transplant osteoporosis, although liver and lung transplant recipients responded more favorably than cardiac transplant recipients.[91]
In cardiac transplant recipients, spine and femoral bone loss were decreased with alfacalcidol plus calcium. Moreover, fewer vertebral fractures were seen in alfacalcidol-treated patients, compared with a control group receiving etidronate and calcium Another study by van Cleemput et al found that 0.25-1 mcg/d of oral alfacalcidol begun approximately 2 weeks after cardiac transplantation improved but did not eliminate bone loss compared with oral etidronate.[92]
De Sevaux and colleagues examined vitamin D metabolites in a cohort of 61 renal transplant recipients and found that nearly half of the patients demonstrated abnormal 1,25-D levels for at least 6 months after transplant, the period associated with the steepest decline in BMD.[93] 1,25 –Dihydroxyvitamin D (1,25D) levels were low at transplantation in all patients and remained subnormal in 64% of patients at 3 months and 47% of patients at 6 months after transplant.
After transplant, the intact PTH levels declined rapidly to just above the normal range. From 3 months after transplant, the 1,25-D levels correlated with creatinine clearance.
Renal transplant recipients receiving alfacalcidol (0.25 mcg/d) with calcium over 6 months had diminished bone loss at the LS and trochanter and almost complete prevention of bone loss at the femoral neck.
Although there were unexpected differences in baseline BMD between the treated and untreated groups in this study, additional analysis of the data suggested that these differences could not explain the results. Severe hypercalcemia was slightly more common in the alfacalcidol group, although nearly all such patients had a high-normal calcium at study entry. Urinary calcium was slightly higher also in the alfacalcidol group at 6 months after renal transplant.[93]
In addition, alphacalcidol may prevent fractures due to falls by improving muscle power.[94]
Kidney transplant and SPKT patients may continue to require posttransplant calcitriol for a brief period at doses lower than used during dialysis.[95] However, therapy must be individualized because a significant proportion of patients have persistent hyperparathyroidism, and calcitriol could worsen hypercalciuria and hypercalcemia.
Studies of oral calcitriol in solid organ transplantation have yielded mixed results. Spinal bone loss was not prevented with low dose of calcitriol, 0.25 mcg/d or 0.5 mcg/48 h in heart and kidney recipients.[66] In a single center in Spain, Toro et al described significant improvement at the femoral neck with alendronate and calcitriol administered late in the postoperative course. After approximately 13 months of treatment, a significant increase in BMD at the femoral neck was seen, although no improvement was seen at the level of the spine.
Begun immediately after heart or lung transplantation, 6 months of calcitriol at 0.5 mcg daily versus cyclic etidronate was associated with spine and femur neck bone loss, although less than in an untreated historical control group.[96] This benefit clearly did not persist beyond 12–24 months.
In a randomized double blind 2-year study using higher doses of calcitriol at 0.5-0.75 mcg daily, beginning by 4 weeks posttransplantation, randomized to placebo or 12-24 months of calcitriol, similar spine bone loss occurred in all groups, but there was less femur neck bone loss by 1 year with calcitriol. As in other studies, the benefit of calcitriol waned after its discontinuation.
As might be anticipated with this activated form of vitamin D, hypercalcemia and hypercalciuria were common, seen in 18% and 59% of patients treated with calcitriol. Routine monitoring of urine and serum calcium is indicated if calcitriol is prescribed.
Calcitriol may have significant nonosteogenic benefits, which include recognized immunomodulatory and steroid-sparing actions. In a Turkish study of renal transplant recipients, patients who received calcitriol had lower PTH levels in the third year posttransplantation, as well as decreased requirements for pulse steroid doses. The increase in creatinine levels was also less in the calcitriol group. The authors concluded that calcitriol may reduce the rate of loss of renal function after renal transplant and protect renal allograft function.[97]
Vitamin D and calcium alone are clearly insufficient to prevent transplant-related bone loss.[98] Bisphosphonates are clearly the drugs of choice for steroid-induced osteoporosis.[84] Although the sun is the major natural source of vitamin D, unnecessary exposure to ultraviolet light cannot be recommended because of the increased incidence of skin cancers in transplant recipients.
Hypogonadism is common and frequently untreated in this medically complex population. Consider hormone replacement (estrogen or androgen) if evidence of hypogonadism exists, if not medically contraindicated.
Unfortunately, the literature regarding medical therapy to prevent or treat transplant-associated bone loss is plagued by relatively small numbers of patients with insufficient power to detect significant differences in BMD, differing immunosuppressant regimens, no randomization, or randomization at varying intervals following transplantation. This is particularly important since it is not appropriate to compare interventions in the early posttransplant period (within 6 mo of transplant) when bone loss is greatest, with later interventions. Moreover, the vast majority of studies are not powered to detect fracture outcomes.[66]
In humans, a pilot study in heart transplant recipients demonstrated a benefit of bisphosphonates (both PO and IV) plus oral calcitriol versus calcium and oral vitamin D. The antiresorptive group of 18 patients received 1 dose of intravenous pamidronate at 60 mg within 2 weeks of transplant and calcitriol at 0.25 mcg/d. This was followed by cyclical etidronate at 400 mg/d for 14 days every 3 months. A second group received similar calcium and vitamin D but no antiresorptives.
After 12 months, spinal BMD was maintained in the group receiving bisphosphonates and calcitriol, whereas the comparator group lost 6-7%. Over this same period, femur-neck BMD fell 2.7% in the antiresorptive group, while the comparator group lost 10.6%.[99]
Valero et al demonstrated a benefit of both etidronate and calcitonin in 120 patients after liver transplantation. At 1-year posttransplant, 35% had documented osteoporosis. Patients received calcium at 1000 mg/d and cyclical etidronate 400 mg orally for 15 days every 3 months or calcitonin, 40 IU intramuscularly daily. After 1 year, this uncontrolled study showed significant improvements in vertebral BMD in both groups (6.4% and 8.2%, respectively).[100]
In a controlled, nonrandomized, nonblinded study from the University of North Carolina in 34 cystic fibrosis patients after lung transplantation, patients receiving intravenous pamidronate gained approximately 8.8% BMD at the LS spine and 8.2% at the femur over 2 years.[101] Pamidronate at 30 mg intravenously was administered every 3 months together with vitamin D at 800 IU/d and calcium at 1000 mg/d. The pamidronate group was compared with a similar group receiving vitamin D and calcium alone. Although the study was not powered to detect differences in fracture prevalence, intravenous pamidronate was significantly more effective than placebo in improving BMD.
The rapid early bone loss during the first 12 months following renal transplant can be prevented by intravenous pamidronate. In a prospective, randomized, controlled study, 26 male renal transplant patients received either placebo or intravenous pamidronate at 0.5 mg/kg at the time of transplant and 1 month later. All patients received prednisolone, cyclosporine, and azathioprine. Patient profiles were similar, as were PTH levels. With transplantation, similar decreases in serum creatinine levels were observed in both groups.
At 12 months posttransplant, spine and femur-neck BMD were preserved in the pamidronate group, whereas in the control group, spine and femur-neck BMD fell 6.4% and 9%, respectively. In this small study, transient hypocalcemia was the only noted adverse effect, seen in 2 patients.[102]
Haas et al demonstrated the ability of a third-generation bisphosphonate, zoledronic acid, to prevent bone loss in the first 6 months after renal transplant.[103] In a randomized, placebo-controlled study, 20 renal transplant recipients received either 4 mg of zoledronic acid or placebo twice within 3 months posttransplant.
In addition to BMD by DEXA, mean trabecular calcium and trabecular morphometry were assessed by bone biopsy. Renal function did not change after zoledronic acid infusion. Two IV infusions of zoledronic acid prevented bone loss and increased average trabecular calcium concentration significantly over the first 6 months after transplant, compared with placebo group in which no change was seen.
BMD at the femoral neck showed no change in the zoledronic acid group but fell in the placebo group. BMD at the lumbar spine increased in the zoledronic acid group and was unchanged in the placebo group. Improved trabecular mineralization and architecture despite ongoing use of high-dose steroids was notable. Cancellous bone was stabilized. An increase in osteoid surface was seen and osteomalacia excluded based on bone turnover markers, although tetracycline labeling was not performed.
The increase in bone mineralization density distribution with zoledronic acid suggested increased trabecular mineralization and argued against osteomalacia. Adynamic bone disease was excluded on the basis of increased osteoid in the zoledronic acid group.
Disappointingly, the early bone-sparing effects of short- term zoledronic acid conferred no sustained benefit compared with placebo at 3 years after transplantation.
Giannini et al reported on the ability of alendronate to prevent further bone loss in renal transplant recipients.[95] The patients’ initial BMD scores were depressed, suggesting at least osteopenia at all measured sites (ie, lumbar spine, total femur, and femoral neck). During a 12-month period of observation, while subjects received 980 mg of dietary calcium, BMD fell further. Bone density decreased at the spine, total femur, and femoral neck.
Subjects were then randomized to alendronate plus calcitriol and calcium versus calcium and calcitriol alone. After 12 months of calcium 500 mg/d with calcitriol at 0.5 mcg/d, no trend toward further bone loss was noted. However, after 12 months of alendronate therapy at 10 mg/d plus calcitriol at 0.5 mcg and calcium at 500 mg, bone density increased 5% at the LS spine and 4% at the femur.
Another study demonstrated that the rapid, severe bone loss associated with heart transplantation could be attenuated by either of two preventive regimens. Both nasal salmon calcitonin at 200 U/d with continuous calcitriol at 0.5 mcg/d or intermittent pamidronate at 0.5 mg/kg intravenously every third month were equally effective by 18 months, although initially, pamidronate slowed bone loss more.[104]
A randomized, controlled clinical trial comparing oral clodronate with intranasal calcitonin for the treatment of low bone mass in 46 patients with osteopenia or osteoporosis after kidney transplantation found that both treatments improved BMD at the LS spine. No adverse effect on graft function was noted, although biochemical exacerbation of secondary hyperparathyroidism was documented.[64]
To date, limited data suggest that pretransplant treatment with bisphosphonates decreases posttransplant fracture risk. If administered prior to liver transplant, intravenous pamidronate prevents osteoporotic vertebral collapse.[105] Similarly, a prospective, uncontrolled pilot study using intravenous pamidronate in lung transplant recipients decreased the fracture rate and preserved bone mass at 1-year posttransplantation. The authors urged that bisphosphonate therapy be started before transplant surgery is contemplated.[106]
A study of bisphosphonate therapy administered to kidney transplant patients after the first posttransplant year found significant bone preservation in the femoral neck in the bisphosphonate group but nonetheless saw no correlation between bone loss at the femoral neck and fracture rates in the study's patients, whether or not they had undergone bisphosphonate therapy.[107] The study's patients, who were retrospectively assessed, underwent BMD measurements approximately 1 year after transplantation and again about 2.5 years after that, with 315 patients receiving bisphosphonate during that interval and 239 receiving no bisphosphonate.
Transdermal estrogen therapy provides protection against osteoporosis in postmenopausal women with liver transplants, as it does in healthy postmenopausal women.[108] Estrogen is also known to improve BMD in women receiving glucocorticoids and to prevent CsA-mediated bone loss in animals.
If estrogen is prescribed together with progesterone, fixed daily doses are preferred over cyclic regimens because estrogen can enhance the hepatic metabolism of cyclosporine, resulting in erratic blood levels. Estrogen alone is probably insufficient to prevent transplant-induced bone loss, particularly in the first year following transplantation.[109]
The National Kidney Foundation (K/DOQI) recommends that if a patient has a BMD t-score of –2 or lower at the time of transplantation or at subsequent evaluations, then therapy with a parenteral amino-bisphosphonate should be considered.[83] There remain significant concerns for the use of bisphosphonates in renal patients with preexisting low bone turnover disease, wherein bisphosphonates could further slow bone turnover and potentially increase fracture rate.[93]
Patients should avoid cigarette smoking and heavy alcohol consumption, both of which are associated with negative bone balance. Adequate nutrition is essential for optimal bone health and essential for overall well-being in transplant recipients. A significant number of patients have compromised nutritional status after successful organ transplantation. Malnutrition has been associated with increased morbidity and higher rates of hospitalization.
Low pretransplant body weight that remains low posttransplant negatively affects bone density. Renal transplant recipients with osteoporosis have lower posttransplant cholesterol and HDL levels (likely attributable to nutritional deficiencies).[97] Global assessment of nutritional status by a certified dietician to detect malnutrition followed by appropriate nutritional interventions may be necessary in the transplant recipient.[110]
Exercise that provides a mechanical load to bone represents an osteogenic stimulus. A 6-month, randomized, controlled clinical trial in heart transplant recipients showed that resistance exercise training in addition to alendronate can reverse glucocorticoid-induced osteoporosis.
In this study, 25 heart transplant recipients were randomly assigned to alendronate 10 mg daily, alendronate plus specific resistance exercises, or a nonintervention control group. Resistance training included lumbar extension exercises performed 1 day per week and 8 variable resistance exercises performed twice per week.
Pretransplantation BMD did not differ between the 3 groups. The control group had ongoing significant losses of BMD after 3 and 6 months. Once alendronate was begun, no further regional loss of BMD was noted. Combined therapy of alendronate with resistance exercise was more effective than alendronate alone in restoring BMD. This combination restored BMD of the whole body, femur neck, and lumbar vertebra to within 0.9%, 2.1%, and 3.4% of pretransplantation levels respectively.[111]
Similar exercise-associated preservation of bone mass was demonstrated in the same population in a prospective study of nasal calcitonin with and without resistance exercise in 18 heart transplant recipients. Lumbar BMD declined to 16.9% below pretransplant levels in the calcitonin-only group, whereas the calcitonin-plus-exercise group achieved BMD results to within 5% of their pretransplant levels by 8 months after transplant.[112]
Regular weightbearing and muscle-strengthening exercises are recommended to reduce the risk of fracture (when medically possible) as first-line therapy for osteoporosis. Improving overall fitness is recommended to minimize the risk of falling. Following transplantation, weightbearing exercise should be resumed as soon as possible, and a prescribed rehabilitation program encouraged.[66]
In the transplant recipient, annual assessments of BMD by DEXA scanning are prudent. Any clinical suggestion of fracture should prompt bone radiographs.
If an antiresorptive agent such as alendronate is used, significant increases in spine BMD may be observed within 1 year. An increase in femoral neck BMD may not be seen until after an average of 4-5 years of treatment with alendronate or risedronate, and probably longer with weaker antiresorptive agents.
Renal transplant recipients represent an unusual population. Bone biopsy may occasionally be required to evaluate for the possibility of adynamic bone disease prior to bisphosphonate therapy.[66]
Given the medical complexity of the typical patient awaiting solid organ transplantation, a referral to an endocrinologist or bone metabolism expert should be considered.
For patient education information, see the Osteoporosis and Bone Health Center and Procedures Center, as well as Understanding Osteoporosis Medications, Heart and Lung Transplant, Kidney Transplant, and Liver Transplant. For further information, see Mayo Clinic - Kidney Transplant Information.
Copyright © www.orthopaedics.win Bone Health All Rights Reserved