

Surgery for spina bifida involves a variety of neurosurgical, orthopedic, and urologic procedures. Surgical procedures include the following:
Without closure of the defect, survival is jeopardized. Closure may become more frequently a prenatal procedure.[1, 2, 3]
Some have viewed the connection between spina bifida and hydrocephalus in a unified sense,[4] such that spina bifida is a consequence of fetal hydrocephalus.
Beyond closure, other needed neurosurgical procedures may include shunting for hydrocephalus, including normal pressure hydrocephalus. Many urologic procedures may be required for the neurogenic bladder.
In addition to neurologic defects, spinal deformity is common in patients with spina bifida and is very difficult to treat. However, correction and fusion are necessary to arrest progression and complications and are prerequisites for successful treatment of lower-extremity deformities and for reduction of impedances to walking and sitting. The literature has failed to document a significant quality-of-life improvement with fusion.[5, 6]
Infection is common, particularly in spina bifida patients with a neurogenic bladder. An increased risk exists with any operative procedure. Retethering of the spinal cord frequently occurs and may manifest urologically and orthopedically. Any suspected change in muscle status, which must be monitored serially, or urologic status may be a sign of retethering.[7]
Spinal deformity reconstruction may be particularly challenging because of posterior element deficiencies. These can lead to instrumentation and fusion failures, infection from the neurogenic bladder, and distal ulcers from insensate skin. Anterior procedures are being combined more frequently with the posterior approach to produce a satisfactory fusion.
Lower-extremity procedures are necessitated by muscle imbalance forces. These procedures, particularly hip reduction, have been the most controversial. Opinions differ as to whether they aid in seating and ambulation. Gait analysis has been used to analyze specific candidates who possess motor strength in the quadriceps in the range of "good plus" and who also have hip dysplasia or dislocation.
Procedures distal to the hip often are similar to those for polio but generally are aimed at maintaining the ability to brace the limb to maximize accessibility and independence. These procedures are directed toward reducing deformity, releasing contractures, and balancing muscle forces from the variable neurologic lesions.
The orthopedic surgeon has a large role in the treatment of patients with spina bifida, in addition to surgical intervention. This includes long-term monitoring of neurologic status, motor strength, and joint range of motion. Because many patients require some degree of bracing, examination for skin irritation and breakdown and observation of ambulation to assess the functional utility of the braces are helpful in detecting any change in condition or the need for treatment modification
For more information on this topic, see the Medscape Reference article Spina Bifida.
In the preantibiotic era, persons with spina bifida were not expected to survive, because the spinal cord and, thus, the central nervous system are exposed in this disease. Those who did survive had severe handicaps. Debate over whether surgical treatment should be offered or whether the disease should be allowed to take its natural course has been resolved in favor of treatment. The results of withheld treatment are well documented, with most patients dying within the first 6-12 months.
Malcolm Menelaus pioneered many advances in the treatment of patients with spina bifida and summarized them in his classic book The Orthopaedic Management of Spina Bifida Cystica.[8]
NextPertinent anatomy includes neural innervation, particularly of the lower extremities. Examination of neurologic deficit helps determine the functional level at which the spina bifida cystica lesion has interrupted function.
The myelomeningocele lesion is unique; its effects are significantly different from those of poliomyelitis, in which anterior horn cells are injured. Thus, with a myelomeningocele, motor function is deficient, but sensation is preserved.
The symptoms are also unlike those of cerebral palsy, in which spasticity is common. This is because the spina bifida lesion is generally flaccid, although in some cases a myelomeningocele may be associated with a measure of spasticity.
See the images below.
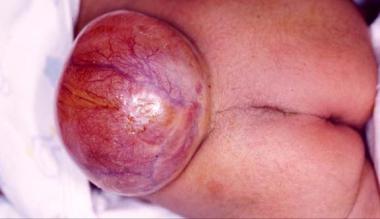 Lumbar region of newborn baby.
Lumbar region of newborn baby.
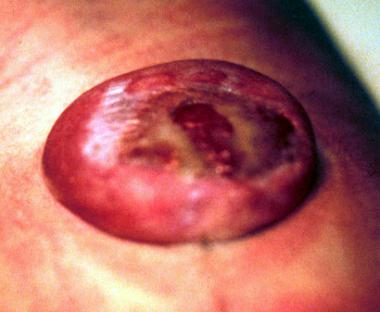 Myelomeningocele in newborn baby.
Myelomeningocele in newborn baby.
At birth, 2 main types of neurologic involvement can be recognized. Type I, which is considered typical, is present in one third of patients in the neonatal period. A certain segmental level is involved, with resulting flaccid paralysis, loss of sensation, and loss of reflexes.
Type II is present in two thirds of patients and is characterized by the interruption of long track signs, with preservation of pure reflex activity, although it may be grossly exaggerated in isolated distal segments. This can present as 3 subtypes, as follows:
In the first subtype, cord function is intact to a certain level, below which a gap is manifested by flaccid paralysis, loss of sensation, and loss of reflexes. More distally, isolated cord function is evident from exaggerated reflex activity.
In the second subtype, the gap in cord function is narrow, virtually amounting to a transection. This condition occurs especially in newborns. There is no movement of the lower limb when the infant is crying, but there is a wealth of purely reflexive activity, including flexor withdrawal, which can be elicited by direct stimulation.
If transection of the long track is incomplete, the child will have spastic paraplegia with preservation of some voluntary movement and sensation. In up to 5% of patients, a hemimyelomeningocele is present, in which one leg is affected with a type I or II lesion and the other may basically be unaffected.
After recognizing the complexity of the actual neurologic deficit, it is clinically useful—because the function and treatment of patients with spina bifida follow broad guidelines—to categorize these patients into general groups. Generally, neurologic levels are grouped as follows:
Independent ambulation generally is a function of having an intact quadriceps muscle with good-plus or excellent-plus strength levels. Patients who do not have adequate quadriceps function may require bilateral Lofstrand crutches or may be restricted primarily to a wheelchair. In such cases, reduction of hip dislocation typically has not been advised because the effect on a person who cannot ambulate is not as consequential as it would be upon a person who can (although some hip dislocations may have a significant impact on a patient's ability to sit properly).
Functional ambulation generally is described according to the following levels, developed by Hoffer and colleagues[9] :
A review from an adult clinic of 84 individuals (minimum age at follow-up, 20 y; mean, 31 y; range, 20-64 y) found that 42 had normal IQs, 70% never needed a neurosurgical shunt, 44% had regular education, 8% had college degrees, 56% were unemployed, 30% lived independently, 23% were either married or divorced with 9 normal offspring, 85% dressed themselves, 65% shopped independently, and 54% drove.[10] Additionally, 31% were thoracic (all used wheelchairs), 12% were L1-L3 (all but 1 used wheelchairs), 33% were L4-L5 (78% used wheelchairs at least part time), and 24% were S1 and below (all walked). Further, 54% experienced decubitus ulcers and, as a consequence, 4 required major extremity amputations. Spinal fusion protected sitting balance, but hip surgery did not produce congruent hips and occasionally resulted in debilitating stiffness; pressure sores resulted in partial foot amputations despite plantigrade feet.
Patients with spina bifida have variable neurologic deficits that characteristically cause deformity as a result of muscle imbalance forces. Unopposed muscle pull can cause spinal deformity, progressive lower-extremity contractures, hip dislocations, and, less commonly, dislocations in other joints.
The assessment of the patient with spina bifida needs to focus on function (ie, accessibility, gait, independence), and treatment should be directed at addressing any deformity that interferes with the patient's potential. When serial follow-up examinations demonstrate worsening function associated with progression of a deformity, surgery should be considered to correct the deformity in combination with release of the deformity and rebalancing of muscle forces around the involved joint as necessary.
Because neurologic impairment is associated with most functional problems, any corrective surgery would presuppose a stable neurologic status. In the patient with spina bifida, a tethered cord may represent a changing neurologic situation that would contraindicate any surgical procedures.
Scoliosis, most commonly paralytic scoliosis, accounts for about 90% of spinal abnormalities in spina bifida.[11] Paralytic scoliosis is characterized by a single, long curve, midthoracic to sacral, often with pelvic obliquity. Paralytic scoliosis is associated with difficulties balancing when sitting and with ischial pressure sores.
Congenital curves may arise at any level and often are associated with vertebral body abnormalities, such as defects of segmentation and formation, as well as mixed defects. Many curves may have associated syringomyelia.
The likelihood of developing a scoliotic curve of more than 30° varies with the level of the myelomeningocele. In spina bifida patients at age 20 years, the approximate prevalence is as follows:
Bracing can be used to allow growth without progression of the deformity, so as to achieve a higher eventual sitting height. However, surgery is indicated for patients with any of the following:
Deficiencies in the posterior elements and in the bone stock generally necessitate an anterior and posterior procedure to achieve a reliable arthrodesis.[12]
Kyphosis of uncommon severity occurs with high-level spina bifida cystica, such that above the T12 level, more than 60% of patients have kyphosis, whereas below that level, only about 10% have a measure of it.[12] Patients with a kyphosis of more than 65° have insidious progression; the extensor muscles become flexors when they migrate to a position anterior to the spinal column and, thus, become a deforming force.
Kyphosis is associated with a high level of chronic skin ulcerations, difficulty sitting, and problems with access for urinary diversion. It can also lead to difficulties in obtaining a properly fitted orthosis. Therefore, kyphosis requires surgical treatment. Unfortunately, some deformities exceed the capability of the anterior abdominal wall to stretch and accommodate a correction. Reducing the deformity, using either excision or a kyphectomy, with the vertebral bodies excised or debulked and collapsed down, allows correction through shortening of the spine.
Excision is more realistic, as an extremely high incidence of failure is associated with implants. A long fusion is necessary, often with segmental attachment and anterior support because of posterior deficiency of the bone from lack of muscle forces and gravitational stresses.
Sacral agenesis may occur in association with a myelomeningocele, and diastematomyelia can be associated with a tethered cord and other complications. Spondylolisthesis may be present, particularly with hyperlordosis. (A common condition, hyperlordosis results from the imbalance of functioning flexors unopposed by the extensors, which have a higher innervation and may be absent.)
Lordosis is never found at birth, but fixed flexion deformities of the hip and lumboperitoneal shunting have been associated with a significant incidence of hyperlordosis. This may be the result of muscle imbalance, particularly pertaining to the hips, or it may be caused by posterior scarring from other spinal surgery. The problem may require an anterior wedge resection or posterior fusion to prevent interference with ambulation.
A sequential study of the lumbosacral angle documented that it did not correlate with tethered cord syndrome.[13] Further, the incidence of spondylolisthesis, which may be associated with tethered cord syndrome and pelvic imbalance, may be treated with in situ fusion in selected cases.[14]
Paralysis at any level may lead to an imbalance of the extensors of the lower extremity, and dislocation of the hip occurs frequently in patients with spina bifida. The incidence increases as the level of the neurologic lesion ascends. Management has varied from ignoring the dislocation to treating it aggressively, but treatment is generally more aggressive in patients with the potential to ambulate and in those with strong quadriceps muscles.
Efforts to reduce the hip in cases of spina bifida require some attempt at muscle balancing to prevent recurrence. In the absence of functioning hip extensors, because their innervation is lower than that of the hip flexors, a posterior-superior dislocation is anticipated. Acetabular dysplasia is seen routinely with disruption of the Shenton line and often with the failure of formation or asymmetry of the ossific nucleus.
In addition to the iliopsoas, which has a tendency to cause a valgus deformity, the adductors may be present and innervated at approximately L3 or L4, causing windblown extremities. In fact, as these present very early, a potential for pelvic obliquity exists. In some cases, a pelvic osteotomy has been recommended to level the pelvis. Traditional indications for hip reduction include a potential ambulation with a strong quadriceps, a good range of motion of the hip, and a level pelvis.
In the 1960s, Sharrard popularized an operation for paralytic dislocation of the hip. In this procedure, the iliopsoas is transferred through a defect created in the ilium, with the muscles balanced by a reattachment to the trochanter for abduction and extension forces.[15]
A progressive dislocation of the hip in a patient who has previously had a stable neurologic status should be investigated, specifically for a tethered cord. If a tethered cord is present, treatment following its release includes the creation of a Staheli-type acetabular shelf, with an external oblique transfer for muscle balancing; this technique has produced good results.
Generally, flexion deformities of the knee occur in patients with weak quadriceps muscles, and only one third of patients with flexion contractures of more than 10° can maintain ambulatory status. These contractures should be braced when they progress past 10-20°, and a release procedure should be performed at 20-40°.
Hyperextension or recurvatum deformity with limited flexion is associated with a poor prognosis for ambulation, especially when it prevents a normal alignment in a young child. The older child may require a quadricepsplasty or femoral osteotomy, particularly in cases of valgus alignment.
The flail foot customarily has no deformity and, for the purpose of function or gravity stress, may be braced to point out at the horizon. The bracing is done early either in a parapodium or in a standing frame, and it is done later in association with other bracing as appropriate. When a varus deformity or equinovarus is noted, it customarily localizes to a neurologic level of L4. This actually is a clubfoot, which usually proves resistant to conservative treatment.
A talectomy may be appropriate in children aged 10 years or younger who have a resistant clubfoot. A triple arthrodesis may possibly be performed after that, and in some cases, calcaneocuboid excision or calcaneal osteotomy may be required.
The calcaneal valgus deformity, which customarily develops after the patient begins weight bearing, interferes with bracing. In some cases of skeletal maturity, a triple arthrodesis may be required.
A calcaneal deformity customarily is localized to lower levels, such as L5 and S1. If left untreated, it progresses to a bulky, prominent heel that is prone to ulceration. At the L4 level, the anterior tibialis is weak, so an anterior capsulotomy and bracing are appropriate. At the L5 and S1 levels, at muscle strength of grade III or better, posterior transfer of the anterior tibialis to the posteromedial calcaneus along with lengthening of the Achilles tendon and a solid ankle-foot orthosis (AFO) may be satisfactory.
A cavus deformity may be associated with a lower neurologic lesion. The lesion is sometimes at S2 and can produce intrinsic tightness, which may respond to a Steindler plantar fascial release from the heel or Dwyer calcaneal osteotomy. At maturity, a triple arthrodesis is very successful.
The equinus deformity develops after birth, secondary to gravity. It customarily is treated by lengthening the Achilles tendon. Equinovalgus with either a pes valgus or vertical talus, which occurs in up to 10% of patients, requires talectomy. For older patients, extensor releases and osteotomy on the lengthening Achilles tendon may be appropriate.
Patients with myelomeningocele can have several complications, of which fracture is very common; 22% of spina bifida patients have a significant fracture in their lifetime. Fracture may occur in association with a significant surgical procedure, such as hip reduction or spine surgery. During the postoperative period, patients are not upright and have disuse osteoporosis complicating their already present osteopenia.
Fracture may occur without pain and often is associated with an elevated temperature and redness, swelling, and warmth in the fracture area. An associated pressure sore sometimes occurs. In some cases, the sedimentation rate and white blood cell count are mildly elevated in reaction to the swelling. In other instances, radiographs reveal marked periosteal elevation and exuberant callus, which occurs because the fracture is adjacent to a Charcot joint. Osteomyelitis and septic arthritis are differential diagnoses.
Management includes inspection of the skin in cases of stable fracture, as well as possible immobilization using existing braces (locked on a 24-hour basis) or plaster immobilization. In some cases, open reduction and internal fixation are required for reasonable function.
Patients who have had a major surgical procedure are best treated as soon as possible, because early treatment may diminish the fracture rate. A study of patients who underwent hip reduction revealed that placing the patient back in some form of cast or hip-knee-ankle-foot orthosis (HKAFO) brace in an upright position in the immediate postoperative period eliminated fracture; prior to the institution of this postoperative weight bearing, 22% of patients sustained fracture on follow-up.
Patients with spina bifida have an increased incidence of sensitivity to latex, which can cause anaphylactic reactions. Anecdotally, the author witnessed an unsuspected reaction during open reduction and internal fixation of a femoral fracture. Although the child undergoing the procedure had had prior operations without incident, upon dissection down to the fracture, anaphylaxis with complete arrest occurred when the author's gloved finger touched the bone. After resuscitation and glove change, the procedure was completed uneventfully.
Risk factors for latex sensitivity, beyond having a myelomeningocele, include multiple operations, particularly in the first year of life. The most common source of latex exposure is balloons, followed by latex gloves. Treatment with latex-free materials has been shown to reduce the incidence of reactions; these materials may also diminish sensitization, and they can familiarize the staff with the allergy problem and with substances that may contain latex that would not otherwise be suspected.
Copyright © www.orthopaedics.win Bone Health All Rights Reserved