

Osteochondrosis is a self-limiting developmental derangement of normal bone growth, primarily involving the centers of ossification in the epiphysis.[1, 2] It usually begins in childhood as a degenerative or necrotic condition. By definition, osteochondrosis is an aseptic ischemic necrosis. Central to the definition is the prerequisite that the epiphysis should have been normal. There is little controversy regarding this requirement, but it is difficult to prove that an epiphysis was normal.
Conditions affecting various sites have been crudely grouped together under the term osteochondrosis. This categorization is primarily based on the morphologic and evolutionary similarities of the involved sites, as seen on radiographs. In broad terms, osteochondroses are a heterogeneous group of unrelated lesions that share the following characteristics:
Practically all of the epiphyses can be affected. Involved sites typically possess many of the features of ischemic necrosis, both radiologically and histologically.
It is prudent to speak of necrosis rather than avascular necrosis because even though ischemia prevails, vessels are present. The vascular theory is controversial because entities such as Blount disease and Scheuermann disease do not lead to ischemic changes and because trauma is implicated as the mechanism for a number of afflictions. However, the ischemic theory should not be abandoned yet, because current investigations have led investigators to a renewed focus on various coagulation-related factors and vascular involvement as the primary culprits.
A key issue in discussing osteochondroses is that the terminology has become increasingly confusing. The term syndrome may be generally preferable to the term disease because it arguably incorporates both the radiographic patterns and the clinical presentations. Some of these conditions, such as Sever disease and Van Neck disease, may be more appropriately referred to as phenomena. In view of the evolving nature of the nomenclature, all 3 terms—disease, syndrome, and phenomena—will be used in this article (see Classification).
Although some clinicians include osteochondritis dissecans among the osteochondroses, this inclusion is controversial. Strictly defined, osteochondritis dissecans is not primarily a disease process involving growth center, and it occurs in adults as well as children. The juvenile form of this process may represent focal ischemic involvement, and only this form should be considered an osteochondrosis.[3]
The inclusion of posttraumatic osteonecrosis and normal variants of ossification in an expanded classification of osteochondroses has also been controversial. As our knowledge of these conditions has grown, it has proved possible to establish their etiologic bases. In the authors’ view, morphologically similar but etiologically unrelated conditions should not be combined in the same group.
NextPrevious classifications divided the osteochondroses into pressure, traction, and atavistic types (Burrows’s classification) or into compression, tension, and atavistic types (Goff’s classification). These systems were inadequate. Siffer proposed a classification that divided osteochondroses into articular, nonarticular, and physeal types; this schema is largely accepted today.
Articular osteochondroses exhibit the following characteristics:
Nonarticular osteochondroses occur at the following locations:
Physeal osteochondroses involve the following:
The use of eponyms for the osteochondroses is so deeply ingrained in the literature that any discussion of these syndromes would be incomplete without the mention of these terms (see the table below).
Table 1. Eponyms for Osteochondroses (Open Table in a new window)
Center Location Eponym Primary Carpal scaphoid Preiser disease[5] Lunate Kienböck disease Medial cuneiform Buschke disease Patella Köhler disease Talus Mouchet disease Tarsal scaphoid Köhler disease Vertebral body Calvé disease (Legg-Calve-Perthes Disease) Secondary Vertebral epiphysis Scheuermann disease and Scheuermann kyphosis Iliac crest Buchman disease Symphysis pubis Pierson disease Ischiopubic junction Van Neck disease or phenomenon Ischial tuberosity Valtancoli disease Calcaneal apophysis Sever disease or phenomenon Accessory tarsal navicular or os tibiale externum Haglund disease Second metatarsal Freiberg disease (or Freiberg infarction) Fifth metatarsal base Iselin disease Talus Diaz disease Distal tibial epiphysis Lewin disease Proximal tibial epiphysis Blount disease[6] Tuberosity of the tibia Osgood-Schlatter disease Secondary patellar center Sinding-Larsen-Johansson syndrome (Sinding-Larsen disease, jumper’s knee) Lesser trochanter of the femur Monde-Felix disease Greater trochanter of the femur Mandl or Buchman disease Capital epiphysis of the femur Legg-Calve-Perthes disease Phalanges Thiemann syndrome Metacarpal heads Mauclaire disease Proximal epiphysis of the radius Schaefer disease Distal epiphysis of the ulna Burns disease Medial humeral condyle Froelich disease Lateral humeral condyle Froelich disease Capitellum of the humerus Panner disease (see Little League Elbow Syndrome) Humeral head Hass disease Clavicle Friedrich diseaseThe initial events in the pathogenesis of osteochondrosis remain elusive, but clinical and radiologic evidence points to ischemic necrosis of the ossification center. This process could be due to a primary vascular event, a definite traumatic event, or multiple additive traumas.
The osteochondrotic process is essentially degeneration of the epiphyseal osseous nucleus. This process is almost certainly due to either (1) interference with the blood supply, which leads to necrosis of the cartilage-canal vessels in the subchondral bone and adjacent epiphysis, or (2) failure of the bony centrum to enlarge and disordered proliferation of the cartilaginous cells in the epiphysis. Secondary changes (eg, fragmentation, collapse, and sequestrum formation) develop in accordance with the characteristics of the affected region.
The disease process can be sequential or simultaneous. It can involve a single epiphysis (isolated disease) or several (multiple-site disease), and not even the sesamoids are spared (as in Sinding-Larsen syndrome and involvement of first metatarsal sesamoids). The underlying processes seem to be essentially the same for isolated and multiple-site disease. However, presentations may vary, depending on the stresses and strains to which the epiphyses have been subjected. The efficacy of regeneration and repair determines the patient’s long-term clinical outcome.
With complete healing, little lasting harm done. However, partial healing or failure to heal becomes a source of chronic pain and disability later in life. Accordingly, the physician must be vigilant to avoid overlooking an osteochondrosis in a pediatric patient.
Ponseti proposed that the following features are common to all osteochondroses:
Duthie and Houghton proposed the following models for the development of osteochondroses:
Other proposed pathogenetic factors for which further study is warranted include an altered collagen-to-proteoglycan ratio,[7] biochemical abnormalities (eg, altered expression of matrix metalloproteinases [MMPs] such as MMP-1, MMP-3, and MMP-13[8] ), and overexpression of glycosaminoglycans and aggrecan as a consequence of altered mechanics that exacerbate damage to the cartilage.
Various investigators have found that major genitourinary disorders are associated with Perthes disease. The risk of inguinal hernia increases 8 times in patients with this disease. Slippage of the capital femoral epiphysis may occur in patients with Scheuermann disease.
Considerable attention has been directed toward the occurrence of growth retardation with osteochondroses. Substantiating evidence for this association includes reduced excretion of urinary deoxypyridinoline and glycosaminoglycans, as well as low plasma levels of insulinlike growth factor-1 (IGF-1). These changes cause a disturbance in the metabolism of collagen.[9] In the future, this alteration may be linked to the pathogenesis of osteochondrosis in syndromic terms.
Osteochondroses should generally be thought of as syndromes rather than diseases because multiple causes—some indeterminable—may be involved. Accordingly, the etiology is largely a matter of hypothesis. The main concern is to identify any distinct pathologic condition present that may be controlled or even corrected with proper therapy.
Various factors have been proposed as potential causes of osteochondroses. The oldest, most controversial, and therefore least widely accepted of these are social deprivation, dietary deficiency, and passive exposure to smoke (an unknown industrial factor). The studies that proposed these factors as causes were geographically specific, and their results might have been confounded by the etiologic fallacy.
The factors currently accepted as the most probable causes of osteochondrosis—alone or in various combinations (in multifactorial disease)—are these:
With respect to genetic predisposition, Blount disease is known to be inherited in an autosomal dominant pattern; however, inheritance patterns of other potentially inheritable disorders (eg, Scheuermann disease[10] ) remain to be characterized.
An area deserving of further study is the genetic predisposition that produces a hypercoagulable state due to a deficiency in tissue factor pathway inhibitor (TFPI).[11] Others include fibrinolysis defects involving protein S, protein C deficiency,[12] and resistance to activated protein C. Likewise, there is no consensus regarding the inheritable disorders of thrombophilia due to mutations in the genes for prothrombin (mutation G20210A), factor V Leiden (mutation G1691A), methylene tetrahydrofolate reductase (C677T mutation), or anticardiolipin antibodies.[13, 14]
With respect to both genetic predisposition and environmental factors, exposure to second-hand smoke may be related to the development of Perthes disease due to the G-455-A polymorphism of the beta-fibrinogen gene.
Deficiencies in trace elements (eg, copper and zinc) have been proposed as probable causes on the basis of animal studies.[15]
Infection, which at one time appeared to have been unanimously discredited as a cause of osteochondrosis, has now been shown to trigger or potentiate the disease process. Its effect may be direct or related to autoimmune mechanisms.
Individual mechanical factors may be related to the development of specific diseases such as Osgood-Schlatter[16, 17] disease and Sinding-Larsen-Johansson disease. Examples of such factors are a long patella (Grelsamer type II) and extensor apparatus and external tibial torsion. Various authors have suggested that Osgood-Schlatter syndrome is traumatic in origin and that it does not involve ischemic necrosis.
Compounding factors associated with osteochondrosis have also been identified. These include hormonal imbalance (hypothyroidism), sickle cell anemia, Gaucher disease and mucopolysaccharidoses, tetany due to magnesium deficiency, and cystic fibrosis, among others. However, all of these conditions are now well-established diseases on their own and, in the authors’ view, should not be linked with osteochondroses.
The frequency with which osteochondroses affect different sites varies. Because they are self-limiting disorders, they often go undiagnosed; consequently, exact documentation is difficult. Perthes disease is considered to be the most common disabling osteochondrosis, but it is not the most common of all osteochondroses. Some osteochondroses occur so infrequently that a physician may never encounter them in his or her whole period of practice.
Most osteochondroses occur shortly after the bony nucleus appears, around the middle of the growth spurt. At this time, the epiphysis is mainly cartilaginous and growing rapidly; therefore, it is susceptible to injury. Exceptions to this general statement include osteochondritis dissecans, Scheuermann disease, and Osgood-Schlatter disease, which predominantly occur during the adolescent growth spurt.
Freiberg disease is more common among females than among males, and juvenile osteochondritis dissecans of the elbow (capitellum) is common among female javelin throwers. All other osteochondroses occur most frequently in males. The delayed appearance and maturation of the growth center in boys may account for this difference. In addition, the high activity level further traumatizes the immature epiphysis.
Some of the common and well-studied osteochondroses have definite racial and ethnic differences in incidence and prevalence. For example, Perthes disease is uncommon among persons of African or Chinese descent, whereas Blount disease is common in Africa but uncommon in Western Europe and North America.
The presentation and findings of patients with osteochondrosis depend on the site of involvement and on the stage of to which the syndrome has progressed.
Pain that is localized to the affected site is usually present in the initial stages (eg, pain during kneeling in patients with Osgood-Schlatter disease or referred pain to the knee in those with Perthes disease). However, clinicians should remember that patients are asymptomatic more often than not and that they typically present late, after the onset of disability. Nonspecific bone and joint pains that occur in children during growth spurts are often overlooked, and many of these may reflect osteochondroses.
Findings commonly found alone or in combination include the following:
Growth disturbance and secondary deformities are late presentations in specific entities, such as Blount disease (shortening and tibia vara), Scheuermann disease (kyphosis), and Perthes disease (shortening and coxa vara, magna, or brevis).
Of greatest importance is that systemic symptoms of inflammation (eg, fever, malaise, weight loss, local redness, and raised temperature) should alert physician to search for causes other than an osteochondrosis.
The differential diagnosis of osteochondrosis includes the following entities:
Osteochondroses, by themselves, do not primarily alter laboratory parameters. However, it is imperative to rule out other disorders before an osteochondrosis is diagnosed.
A complete hemogram is mandatory. It should include the erythrocyte sedimentation rate (ESR) or C-reactive protein (CRP) level and blood chemistries, with a particular focus on serum calcium, phosphate, and alkaline phosphatase levels. Other laboratory investigations depend on the patient’s individual case.
Studies to detect or evaluate genetic polymorphisms, trace elements (copper, zinc), urinary deoxypyridinoline excretion,[18] and plasma insulinlike growth factor-1 (IGF-1) levels are primarily of research interest and should not be used to screen patients.
Most osteochondroses were first described in terms of radiographic appearances, and for the most part, they continue to be characterized on this basis. Although newer investigations such as magnetic resonance imaging (MRI) and bone scintigraphy may help in early identification of the disease, these studies are seldom feasible, because patients typically do not exhibit symptoms in the early stages of disease.[19]
Some radiographic features reflect specific entities, whereas others are shared among the osteochondroses (see the images below).
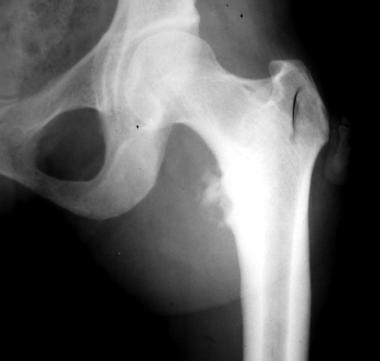 Osteochondrosis of the lesser trochanter of the left femur (Monde-Felix disease). Image shows fragmentation.
Osteochondrosis of the lesser trochanter of the left femur (Monde-Felix disease). Image shows fragmentation.
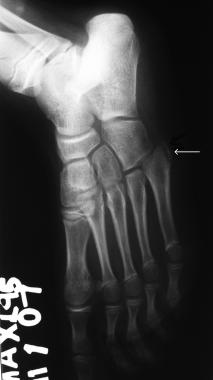 Osteochondrosis of the base of the fifth metatarsal bone (Iselin disease). Image shows fragmentation.
Osteochondrosis of the base of the fifth metatarsal bone (Iselin disease). Image shows fragmentation.
Early involvement typically results in a centrum that appears to have reduced size, increased opacity, and irregular architecture. In addition, images may depict asymmetry in the size of bony trabeculae, as well as irregular trabeculation.
With advancement, fibrillation and fissures in the cartilage enlarge, and sequestrum formation may or may not occur. The crescent sign (ie, separation of part of the bone from a parent nucleus) may be present. In weight-bearing joints, the joint space may be reduced, but this finding is unusual.
Revascularization usually manifests as worsening of the radiographic picture, with the development of osteoporosis, absorption of necrotic tissue, and deformation of epiphysis due to a loss of underlying support (ie, step defect and buttressing phenomenon).
The picture in the squeal or burnt-out phase depends on the degree of ischemic damage and its chronologic arrest. Complete and early restoration by reparative processes leaves no deformity. By comparison, late and incomplete restoration of the blood supply—compounded by additional assault and poor protection—develops into an irregular, deformed, and misshaped epiphysis. Associated findings may include collateral and subsequent changes in the metaphysis and joint.
Specific deformities that may result include the following:
Joint arthrosis is usually the delayed presentation of all of the articular osteochondroses (eg, Kienböck disease, Perthes disease) that are poorly restored. Its causes are an abnormal distribution of stresses and an irregular joint surface.
MRI may be helpful in diagnosing an osteochondrosis before obvious radiographic changes become apparent. It is also useful for differentiating osteochondrotic diseases from inflammatory conditions, such as tubercular involvement and osteomyelitis.
Physeal mapping of the growth plate can be undertaken to facilitate surgical correction (eg, of Blount disease). MRI can reveal small fragments better than other imaging studies can. It can also help elucidate residual attachments to parent bone and vascularity in cases of juvenile osteochondritis dissecans, Freiberg disease, Sinding-Larsen-Johansson disease (jumper’s knee), Osgood-Schlatter syndrome, and other osteochondroses. Moreover, associated information, such as the presence and extent of underlying bone defects, can be judged on MRIs.
Early MRIs show focal hyperintensities in the epiphysis or neighboring soft tissues; these are due to edema. Variable signal intensity is seen in the avascular fragment; edema is hypointense and sclerosis hyperintense on T1-weighted images. Marrow edema in the parent bone is a regular finding. Changes in signal intensity can range from peripheral irregularity of the ossific nucleus to complete replacement of the normal marrow fat (see the image below). Intra-articular effusion may also be seen.
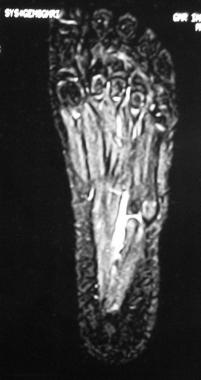 MRI in the same patient as in image above shows altered marrow signal intensity in the apophysis of the fifth metatarsal base. This finding is suggestive of Iselin disease.
MRI in the same patient as in image above shows altered marrow signal intensity in the apophysis of the fifth metatarsal base. This finding is suggestive of Iselin disease.
Revascularization leads to replacement of the necrotic focus with fatty marrow. Loose bodies and residual deformities are common in osteochondritis dissecans but rare in osteochondroses. In Scheuermann kyphosis, Schmorl nodes can demonstrate low or high signal intensity on T2-weighted MRIs in association with neighboring marrow edema. Disk herniations and diskogenic sclerosis are also observed.
Likewise, in Köhler or Freiberg infarction, frequent findings include deformity, fragmentation, fractures, collapse, and edema. Perthes disease may result in acetabular labral tears and metaphyseal cysts, in addition to the aforementioned features.
Bone scintigraphy has been shown to be highly sensitive and specific in detecting avascularity. Whether it is better than MRI for this particular application is still the subject of considerable controversy.
Scintigraphic changes in Perthes disease have been extensively studied; however, scintigraphic changes in other osteochondroses continue to be neglected, probably because these conditions are relatively uncommon. In Perthes disease, the preferred study is pinhole imaging with both anteroposterior and lateral views obtained by using a technetium-99m tracer (see the images below).
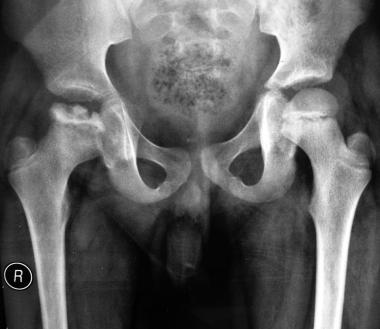 Perthes disease. Anteroposterior view of the pelvis and both hips. Image shows pathology involving the right hip joint. Also depicted is flattening, fragmentation, and sequestrum formation affecting the capital epiphysis of the femur, with metaphyseal cyst formation and osteopenia. Of note, no subluxation of the hip joint is observed.
Perthes disease. Anteroposterior view of the pelvis and both hips. Image shows pathology involving the right hip joint. Also depicted is flattening, fragmentation, and sequestrum formation affecting the capital epiphysis of the femur, with metaphyseal cyst formation and osteopenia. Of note, no subluxation of the hip joint is observed.
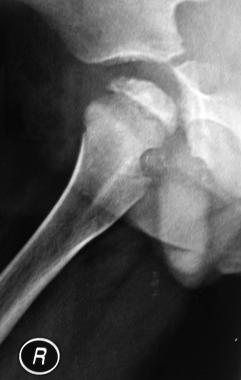 Perthes disease. Lateral view of the same patient as in image above shows flattening of the epiphysis, as well as sclerosis with deformation of the femoral head.
Perthes disease. Lateral view of the same patient as in image above shows flattening of the epiphysis, as well as sclerosis with deformation of the femoral head.
In the initial stages of ischemia, even before any radiographic changes are seen in a symptomatic patient, bone scans show a striking absence of tracer uptake. This absence may be noted either focally or in the entire epiphysis. Uptake in the physis and acetabular rim is normal.
These findings represent growth disturbance in the epiphysis when metabolic activity, and obviously blood flow, are low, before the beginning of a reparative response from the surrounding physis or metaphysis. In this way, bone scanning can help in making an early diagnosis months before radiographic changes become obvious.
As a reparative response is mounted in the form of revascularization from neighboring uninvolved epiphyses and metaphyses, activity at the margin of the hypoactive, necrotic focus increases, and the focus itself gradually decreases in size. Also observed is increased activity in the neighboring physis and metaphysis; this finding represents an osteoblastic response.
A normal distribution of the tracer in the entire epiphysis, as compared with the opposite side, heralds complete healing. In rare cases, degenerative changes can account for the increased activity.
Bone scans may be useful in assessing acetabular containment of the head, sclerosis, subchondral fractures, intra-articular effusions, and fragmentation of the chondral surface. Disappearance of previous activity (eg, from the lateral column in Perthes disease) indicates collapse or infarct. This is a poor prognostic marker. Schmorl nodes show increased tracer uptake in the acute phase and normal or increased activity in late phases.
In general, all stages of the disease process have been described as involving the following histologic features:
The features listed above are not specific to any particular entity and generally resemble those of ischemic necrosis.
The collagen-to-proteoglycan ratio is reduced, as demonstrated by means of electron micrographic studies.
The most important goal in the treatment of articular osteochondrosis is to obtain a congruous, mobile, and painless joint. Nonarticular osteochondroses usually heal with protection. However, concern about undesirable results should prompt the treating physician to provide supervised treatment rather than neglect. Controversial entities, such as Sever disease and Van Neck phenomenon, should be treated if patients are symptomatic. They are not always labeled normal variations in ossification.
To avoid medical and legal pitfalls, physicians must ensure that both patients and parents understand the nature and natural history of the disease, the uncertainty and likely outcome of treatment, and the patient’s prognosis and expectations. It is acceptable to acknowledge and disclose that the current scientific knowledge of the osteochondrotic syndromes is inadequate and that physicians’ understanding of how to recognize and treat these conditions is therefore limited.
The basic principles of therapy for osteochondrosis are as follows:
In general, treatment options for osteochondrosis can be divided into nonsurgical and surgical interventions (see below). Medical and supportive therapies constitute the mainstay of treatment for osteochondroses. Surgery is indicated only for specific purposes, such as replacement of failed conservative treatment, alleviation of symptoms, or reducing late disability. Although the course of an osteochondrosis is protracted, it is generally self-limited. Therefore, it is prudent is to allow the patient’s reparative mechanisms sufficient time to act in an apposite environment.
Osteochondroses frequently resolve completely or partially in response to symptomatic nonsurgical treatment. The main aim is to reduce morbidity and decrease the duration of symptoms with supervised treatment. Still, medical treatment may not influence the final outcome, and this possibility must always be explained in detail to patients and their parents.
To date, no mechanism has been found by which the disease process can be arrested. Close follow-up in the initial phase, with specific interventions guided by the principles mentioned above, remains the standard of care. The treatment plan must be individualized to the specific case. Specific measures can be carried out in accordance with the aforementioned principles of therapy.
The following measures are recommended to help prevent additional trauma:
The following measures are recommended to help prevent secondary deformity and to reduce mechanical stress across the joint:
The following measure is recommended to facilitate reossification:
Antibiotic therapy was formerly employed in the treatment of osteochondroses. Currently, however, the use of antibiotics (specifically tetracyclines) for this purpose is not recommended.
Future therapies that may help to limit the progression of individual mechanisms in Perthes disease are inhibition of receptor activator of nuclear factor-kappa B ligand (RANKL),[20] immunomodulation of T helper cells, and administration of anti–tumor necrosis factor-alpha (anti-TNF-alpha) antibodies.
Administration of bisphosphonates (eg, zoledronic acid) has shown promising results. However, concerns include the possibility of subsequent alteration of morphology and disturbance of physeal growth.
Surgical treatment is usually undertaken when conservative methods are ineffective or failing or when they are expected to fail during follow-up. Another indication for surgical therapy is a situation where such therapy may help restore the reparative process or guide this process in such a way as to improve outcomes. In addition, surgery is sometimes performed to enhance the patient’s cosmetic appearance.
Although full recovery after surgery is uncommon, it has been known to occur even in advanced cases, given the appropriate setting. Therefore, surgery is best deferred until late in the course of the disease, or else reserved for the treatment of disability.
Listed below are some of the generalized procedures that have successfully been used to manage common osteochondroses. They range from minimally invasive procedures to salvage operations.
Arthroscopic procedures are usually performed to manage large joints, with the following objectives:
Open removal of a loose fragment (ossicle) is performed to treat secondary pseudoarthrosis of Osgood-Schlatter disease.
Refixation, either with bioabsorbable pegs or pins (eg, polyglycolide) and screws (preferable) or with autologous bone pegs of partially avulsed patellar tendons, is performed to manage Osgood-Schlatter disease or Freiberg disease.
Osteotomy is performed for the following purposes:
Shelf acetabuloplasty (labral support) procedures include the following:
Salvage surgeries and reconstructive procedures are sometimes required to reduce disability in late cases with poor final outcomes. They include the following:
The surgical approach for each case must be individualized and based on sound principles. In weighing the benefits and risks of any procedure, clinicians should always remember the Salter aphorism: Decision is more important than incision. Some of the newer procedures are still unproven and should not be accepted just because other alternatives may seem to have undesirable aspects.
Regular, vigilant follow-up is required after surgical intervention to promote healing and to maintain the results achieved. Protected mobilization followed by graduated functional activity is necessary whether the osteochondrosis is treated conservatively or surgically at any stage. Follow-up is long, extending many years until skeletal maturity is achieved; otherwise, progress cannot be ascertained. Because the disease may flare up or worsen, a different intervention may be required at some point.
If the anatomy and physiology of the joint are restored after skeletal maturity, the risk of persistent morbidity is low. However, poor restoration is common because of secondary osteoarthritis and related disability. If these sequelae arise, appropriate intervention is necessary.
A guided physiotherapy program must be simultaneously undertaken during rehabilitation with the aim of reducing disability.
Because osteochondrosis is a self-limiting disorder, the outcome is usually good.[22] More often than not, in fact, the syndrome goes unnoticed. However, when osteochondrosis does not remit with conservative treatment or surgery, the patient’s prognosis is usually poor. In these cases, patients may need salvage interventions or joint replacement later in life to manage secondary changes. Patients should be properly informed and educated before such interventions are undertaken.
Copyright © www.orthopaedics.win Bone Health All Rights Reserved