

Infection, though an uncommon complication of arthroplasty, may be among the most devastating complications for the patient, as well as for the surgeon. The economic consequences associated with treating periprosthetic infections are substantial.[1, 2, 3]
Revision procedures for infection are associated with a longer operating time, greater blood loss, and more frequent complications, along with increases in the total number of hospitalizations, duration of hospitalization, total number of operations, total hospital costs, and total outpatient visits and charges. When compared with revisions for aseptic loosening or primary total hip arthroplasties (THAs), revisions for sepsis are associated with significantly greater use of hospital and physician resources.[3]
Currently, the reported infection rate after arthroplasty is about 1%.[4] Kurtz et al quantified the current and historical incidence of periprosthetic infection associated with hip and knee arthroplasty in the United States using the Nationwide Inpatient Sample, as well as corresponding hospitalization charges and length of stay, and found that the rate of infected knee arthroplasties was 0.92%, significantly greater than the rate of infected hip arthroplasties (0.88%).[5]
The number of primary and revision procedures has been projected to expand dramatically over the next 25 years[6] ; accordingly, the infection burden on patients, clinicians, and society as a whole will also increase. The estimated cost of infected revisions is projected to be as high as $1.6 billion by 2020. Methods of preventing, diagnosing, and treating infection must be continually improved in order to reduce the cost and complications of total joint arthroplasty.
There is a need for both improved diagnostic methods and more efficient treatment protocols. Molecular diagnostic methods, such as polymerase chain reaction (PCR) testing, have been associated with high false-positive rates and are still not recommended for routine clinical use. Addressing bone defects during reimplantation is an area that also requires further research.
The problems of conducting randomized studies in the setting of a septic prosthetic failure are a major deterrent to the evolution of treatment protocols. Foolproof intraoperative diagnostic techniques, improved implant designs, and better local antibiotic delivery systems must be developed to face the menace of infection associated with joint replacement surgery.
Gains may be achieved not only by developing newer approaches but also by using currently available approaches more effectively. For example, in one study, culture of samples obtained via sonication of prostheses was more sensitive than conventional periprosthetic-tissue culture for microbiologic diagnosis of prosthetic hip and knee infection, especially in patients who had received antimicrobial therapy within the 14 days preceding surgery.[7]
In a meta-analysis, antigranulocyte scintigraphy with monoclonal antibodies had a reasonably high discriminating ability with respect to identification of prosthesis infection in patients who underwent total joint arthroplasty.[8]
The use of cefuroxime-impregnated cement was shown to be effective in the prevention of early to intermediate deep infection after primary total knee arthroplasty (TKA) performed with perioperative systemic antibiotic prophylaxis but without any so-called clean-air measures.[9] This measure may be particularly helpful in developing nations, where more and more arthroplasties are now being performed.
Additional pathogens are being found to cause prosthetic joint infections. A Swiss study drew attention toward infection by Propionibacterium acnes; a median of 10 biopsies, a 14-day incubation period, and histopathologic examination were needed to establish the association.[10]
Thus, the future of management of arthroplasty-associated infections lies in devising ways to prevent infections, developing investigations that allow early detection, and minimizing both the emergence of new pathogens and the spread of antibiotic resistance among existing pathogens.
NextThe Musculoskeletal Infection Society has proposed the following criteria for periprosthetic joint infections (PJIs)[11] :
Tsukayama et al classified arthroplasty-associated infections into four types according to the most common presenting patterns; they also recommended treatments for each type, as follows[12] :
The factors influencing the occurrence of arthroplasty-associated infections include the following[13] :
Debreuve-Theresette et al described the development of a score to asses the endogenous risk of surgical site infection (SSI) after total hip arthroplasty (THA) and total knee arthroplasty (TKA).[14] They found the score useful for identifying SSIs but noted that further validation in larger studies would be required.
Immunocompromised status, rheumatoid arthritis, and diabetes mellitus are some of the most important comorbid conditions associated with an increased risk of infection in a patient undergoing arthroplasty.
Malnutrition has been defined by many authors as a total white blood cell (WBC) count lower than 1500/µL and an albumin level lower than 3.5 mg/dL.[15] Malnourished patients have an increased chance of wound complications.[16] Hence, it is imperative to build up the patient’s nutritional status before the operation.
Obesity is another important factor to be considered. One study found obesity to be an independent risk factor for the development of deep sepsis after hip arthroplasty.[17]
Chronic urinary tract infection and ongoing sepsis in any other part of the body also increase the risk.
Steroid use for an associated condition (eg, inflammatory arthritis or psoriasis) has been associated with increased susceptibility to infection. A previous surgical procedure on the same joint is an independent risk factor that doubles the risk of deep infection in the knee and triples the risk in the hip.[18, 19] Elderly patients and patients with comorbid conditions that require a long time for optimization before surgery are more susceptible to infection as well.[20]
The number of personnel and the amount of traffic in the OR exert an influence on the incidence of infection. Accordingly, changes in the OR environment have the potential to reduce infection. For example, some people shed a large number of bacteria; they can help decrease the risk of infection by using helmet aspirator suits.
Although various commercial preparations are available for preparing the surgical site, most surgeons prefer the iodophor compounds. Shaving the area is best avoided, but if it is considered essential, it should be done immediately before the procedure. The use of an iodophor-incorporated drape has been proved to decrease infection in arthroplasty patients and is now routine in this setting.
In one study, the use of an iodophor-incorporated drape during 649 TKAs was associated with an infection rate of only 0.5%.[21] In another study, the use of an iodophor-incorporated drape proved to be significantly better at preventing recolonization of the skin by bacteria than other methods of skin-site preparation were.[22]
Ultraviolet (UV) light has been employed to decrease the bacterial load in the OR. This measure adds to the cost of the procedure but has not been conclusively shown to produce a decrease in infection risk.[23]
Use of vertical laminar airflow has been found to yield a significant decrease in the bacterial load in the OR air and thereby decrease the infection rates with THAs. In an important study from the early 1980s, Lidwell et al concluded that laminar airflow systems and body exhaust suits independently reduced surgical infection risk by 50%.[24]
However, Miner et al, in a 2007 population survey designed to assess the efficacy of laminar flow and body exhaust suits, found no conclusive evidence to support this result.[25] The cost of laminar airflow systems and body exhaust suits is substantial, adding hundreds of dollars to the expense of each operation and complicating the environment in which the operating team works. The authors concluded that it would be worthwhile to obtain additional evidence about whether these widely used clean-air practices have a meaningful clinical benefit in today's OR.
A prolonged operating time increases the risk of infection.[26] In one population-based survey, a prolonged operating time was in fact the only statistically significant risk factor for infection.[25]
The type of implant used also influences the infection risk. For example, a large hinged knee implant increases the risk of infection. Materials such as polymethylmethacrylate (PMMA), steel, cobalt-chromium (CoCr), and polyethylene (PE) are susceptible to the formation of a biofilm by the infecting organism around the implant, which protects the pathogen from the action of antibiotics.[27]
The virulence of the infecting organisms is an important factor to consider in planning management. Staphylococcus aureus and Staphylococcus epidermidis are the most commonly isolated organisms. Gram-negative infections are resistant to treatment and are a frequent cause of recurrence after an exchange arthroplasty.[28] Organisms that can form glycocalyx, methicillin-resistant S aureus (MRSA), group D streptococci, and enterococci are considered highly virulent.
A frequent cause of inability to isolate the organism is empirical antibiotic therapy. Other causes are infections caused by anaerobic organisms and fungi. Trampuz et al reported the frequencies with which different pathogens cause prosthetic joint infections (see Table 1 below).[29]
Table 1. Microorganisms Causing Prosthetic Joint Infections (Open Table in a new window)
Microorganism Frequency (%) Coagulase-negative staphylococci 30-43 Staphylococcus aureus 12-23 Streptococci 9-10 Enterococci 3-7 Gram-negative bacilli 3-6 Anaerobes 2-4 Multiple pathogens (polymicrobial) 10-12 Unknown 10-11
Prosthetic joint infections are caused by pathogens living clustered together in biofilm, a highly hydrated extracellular matrix attached to the implant surface. Within the biofilm, the organisms grow either slowly or not at all; consequently, they are resistant to all growth-dependent antibiotics. The bacterial cells of the biofilm organize among themselves, communicate with each other, and behave like a multicellular organism. The biofilm helps the pathogens combat external and internal forces, whether from the host immune system or from antibiotics.[29]
There is some evidence to indicate that intracellular internalization of staphylococci may be a mechanism in the pathogenesis of infection and resistance to treatment.[30, 31]
The Surgical Infection Prevention Project and its Surgical Infection Prevention Guideline Writers Workgroup have promoted three major interventions to prevent surgical site infections (SSIs)[32] :
With increasing frequency, patients are admitted to the hospital on the day of the operation and present to the OR just before their procedure. It is therefore essential to ensure that the institution of safeguards regarding the correct surgical site and the administration of prophylactic antibiotics no more than 1 hour before surgery are implemented as components of an institutional process.[33]
The cephalosporins cefazolin and cefuroxime are considered to have equal prophylactic efficacy.[34] Local guidelines that recommend beta-lactam agents as first-line prophylaxis should also recommend alternative drugs for those allergic to penicillin. Available evidence suggests that administering the first dose as near to the incision time as possible will reduce the likelihood of infection, whether superficial or deep.
There remains some controversy regarding the optimal duration of prophylaxis in connection with THA. The US advisory statement recommends that antimicrobial prophylaxis be administered no more than 1 hour before the incision and discontinued within 24 hours after the end of the operation.[32] However, European guidelines recommend administering a single dose no more than 30 minutes before the incision and advise considering prophylaxis for as long as 24 hours after the operation.[35, 36]
In a Dutch study, superficial and deep SSIs occurred in 2.6% of 1922 THA patients, and the highest odds ratios for infection were found in those who received prophylaxis after incision, those with an American Society of Anesthesiology score of 12, and those in whom the operating time was above the 75th percentile.[37] Prolonged prophylaxis after the end of the procedure and the use of antibiotic-impregnated cement did not contribute to fewer infections in this study.
In the presence of wound drainage, erythema, and swelling about the hip associated with systemic symptoms (fever, chills, and generalized malaise), the diagnosis of an infected total hip arthroplasty (THA) can be relatively straightforward . Unfortunately, many periprosthetic infections do not exhibit obvious signs and symptoms of infection.[38, 39]
In the absence of obvious indicators, a thorough history and a detailed examination of the patient should suggest the possibility of infection. A high index of suspicion is necessary. Often, the only symptom is the acute onset of pain in a previously well-functioning joint.
The American Academy of Orthopaedic Surgeons clinical guidelines are helpful for guiding the management of prosthetic joint infection.[40] The Infectious Diseases Society of America has also issued clinical practice guidelines on the diagnosis and management of prosthetic joint infections.[41]
The erythrocyte sedimentation rate (ESR) and the C-reactive protein (CRP) level provide excellent diagnostic information for establishing the presence or absence of infection before surgical intervention in patients with pain at the site of a knee arthroplasty. ESR values higher than 35 mm/hr have been associated with deep infection.[42, 43] However, the ESR is not always elevated in a chronic deep infection. When the ESR is used alone, its reported specificity and sensitivity are 0.82 and 0.86, respectively.
The CRP level usually peaks on postoperative day 2 and falls back to normal levels by 2 to 3 weeks. It is usually normal in cases of aseptic loosening but is elevated by more than 10 mg/L in cases of infection. When used along with ESR, the CRP level has a specificity of 1.00 for diagnosing periprosthetic infections.[42, 43, 44] Repeated measures of the CRP level showing a rising or falling trend are more useful for deciding on management and planning follow-up.
The total white blood cell (WBC) count may be a helpful addition. In fulminant infections, leukocytosis with an increase in immature neutrophils may be noted. In indolent infections, the complete blood count (CBC) with differential may be normal.[4]
Aspiration of fluid from a suspected joint is highly sensitive and specific for periprosthetic infection, and some recommend that it be done routinely in the setting of an abnormal erythrocyte sedimentation rate (ESR) in a young implant.[39]
Aspiration is used to confirm a clinical suspicion of infection or to support or negate the findings of other preoperative investigations, such as the ESR and the C-reactive protein (CRP) level, which may be falsely elevated because of connective-tissue disease. An additional benefit of aspiration in instances of suspected infection is the ability to identify the organism and its antibiotic-sensitivity profile, which may influence preoperative planning and the choice of an antimicrobial agent if use of an antibiotic depot is planned.[42]
Aspiration must be performed under aseptic conditions. Contamination of the sample by skin organisms of and inoculation of organisms into the joint are the main concerns. To increase the chances of culture positivity, antibiotics should be discontinued 2 to 3 weeks before aspiration. Glucose levels and cell counts are obtained, and cultures are done on three samples. If all three samples are positive, the diagnosis is established. If two are positive and blood parameters are elevated, the diagnosis of infection is made; otherwise, the aspiration is repeated.
Parvizi et al found that a calorimetric strip test for leukocyte esterase in the synovial fluid has high specificity and positive predictive value in the diagnosis of prosthetic joint infection.[45] Synovial fluid interleukins 1 and 6 may become valuable indicators of infection.[46]
Histopathologic examination of frozen tissue samples during surgery has a low sensitivity but a high specificity for diagnosing infection.[47] Tissue from the bone-cement or prosthesis-bone interface, as well as from abnormal-appearing areas of synovial tissue, should be examined. The presence of more than five polymorphonuclear leukocytes per high-power field is diagnostic of infection, but the presence of fewer than five does not suffice to rule out infection.[47]
A preoperative core biopsy provides the advantages of aspiration and tissue examination and is a useful investigation in suspected joints.[48]
Plain radiographs should always be obtained when infection in a prosthetic joint is suspected, even though they are rarely diagnostic in themselves. Serial radiographs often aid in diagnosing infection. A particular difficulty is distinguishing septic loosening from aseptic loosening. Periosteal new bone formation may help in differentiating these two conditions; this finding has been associated with the presence of deep sepsis in total hip arthroplasty (THA) and is considered by some to be pathognomonic for this condition.[49]
More severe and longstanding cases periprosthetic infection may show evidence of implant loosening, lysis, bone resorption adjacent to the implant, areas of new bone formation, and periosteal reaction (see the image below).
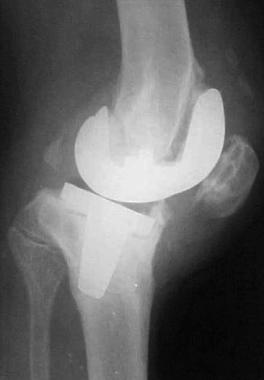 Lateral radiograph showing lysis and loosening of prosthesis.
Lateral radiograph showing lysis and loosening of prosthesis.
Progressive loosening is a worrying sign that suggests potential infection. Endosteal scalloping is highly suggestive of infective loosening. Loosening on the acetabular side is indicated by migration of the cement mantle or the socket (see the image below), protrusion, or fracture. Arthrography, sometimes done in conjunction with aspiration, can help diagnose loosening but cannot confirm infection.
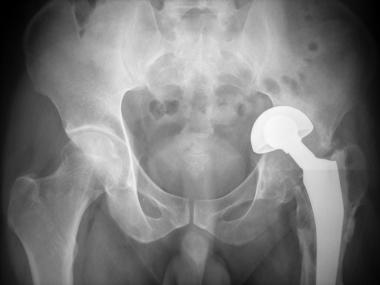 Radiograph of 50-year-old patient for whom bipolar arthroplasty was done elsewhere for displaced fracture of neck of femur. Patient came with severe pain hip 1 year after procedure. Severe osteolysis and superior migration of prosthesis are seen.
Radiograph of 50-year-old patient for whom bipolar arthroplasty was done elsewhere for displaced fracture of neck of femur. Patient came with severe pain hip 1 year after procedure. Severe osteolysis and superior migration of prosthesis are seen.
Technetium-99m (99mTc) and indium-111 (111I) polyclonal antibody scans and monoclonal antibody scans have proved useful in diagnosing arthroplasty-associated infections; however, they cannot distinguish between septic and aseptic loosening. 99mTc scans are sensitive but not specific. Gallium scans are similar to 99mTc scans.
Sequential 99mTc- and 111I-labeled white blood cell (WBC) scanning is highly sensitive and specific and can be used to make the diagnosis in equivocal situations.[50] The study is considered to be positive for periprosthetic infection when there is activity on the labeled leukocyte scan but no corresponding activity in the same area of distribution on the 99mTc scan. Love et al reported a sensitivity of 100%, a specificity of 91%, and an accuracy of 95% when both imaging modalities were used to detect periprosthetic infection at the site of total joint arthroplasty.[51]
Similarly, fluorodeoxyglucose positron emission tomography (FDG-PET) scanning has shown promising results in the majority of studies for the detection of periprosthetic infection.[52, 53]
In managing a septic prosthetic failure, the goals are to eradicate the infection and to provide a functional limb. The various treatment modalities include long-term antibiotic suppression, débridement, exchange arthroplasty, and arthrodesis (see Table 2 below).[29] Amputation is done as a desperate procedure in case of life-threatening sepsis.
Table 2. Concise Approach to Treatment of Prosthetic Joint Infections (Open Table in a new window)
Status of Prosthetic Joint Infection Treatment Duration of symptoms < 3 wkThe Tsukayama classification of arthroplasty-associated infection into four types (see Definition and Classification) can help in formulating the treatment recommendations for the infection. Tsukayama et al based their treatment of infections after total hip arthroplasty (THA) on the clinical presentation—that is, positive intraoperative cultures, early postoperative infection, acute hematogenous infection, or late chronic infection. Treatment protocols have been successfully used for these four clinical settings.[28]
Patients who have positive intraoperative cultures can be managed with intravenous (IV) administration of antibiotics for 6 weeks without operative intervention. In these patients, a revision prosthesis has already been implanted for presumed aseptic loosening before the results of the intraoperative cultures become available. Conceivably, this may be a situation for routine use of antibiotic cement in all revisions.
Patients who had an early postoperative infection are managed with débridement, replacement of the polyethylene (PE) insert of the acetabular/tibial component, retention of the prosthesis, and IV administration of antibiotics for 6 weeks.
Removal of the original PE insert permits greater access to the site of the acetabular/tibial component for débridement and removal of a possible nidus of infection. In both the hip and the knee, removal and replacement of the PE liner are frequently necessary to provide access to the complete joint for thorough débridement, and this is the reason why arthroscopic débridement has fallen into disfavor in the knee.
Attempts are made to check the fixation of components manually. There should be no radiologic evidence of loosening. Débridement is tried once or twice, with IV antibiotics administered at an adequate dosage. Although an attempt is generally made to salvage the prosthesis, removal may be warranted in cases where infection is recalcitrant or the implants are loose. Successful results can be attained when the duration of presentation is shorter or when the pathogen is a low-virulence organism.
The success rate of irrigation and débridement in the treatment of early postoperative infection has ranged from 0% to 100%.[54, 55, 56, 57] Nolan et al reported that this procedure was successful for all of six infections diagnosed within 2 weeks after an index total hip arthroplasty.[54] In general, it appears that the longer that the infection has been present in the hip, the more difficult it is to eradicate it without removal of the prosthesis.
Patients who have an acute hematogenous infection are also managed with débridement, replacement of the polyethylene insert, retention of the prosthesis if it is not loose, and IV administration of antibiotics for 6 weeks.
Patients who have a late chronic infection are managed with débridement, removal of all prosthetic components and bone cement, and either a direct one-stage revision[58, 59] or a two-stage exchange (see the images below).[60, 61, 62] Eradication of the infection would entail removal of components in most of these patients, and trying to salvage the prosthesis may be futile.
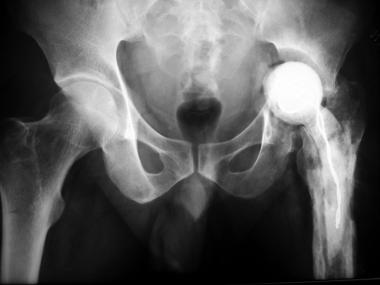 Stage I revision with antibiotic spacer made from mold.
Stage I revision with antibiotic spacer made from mold.
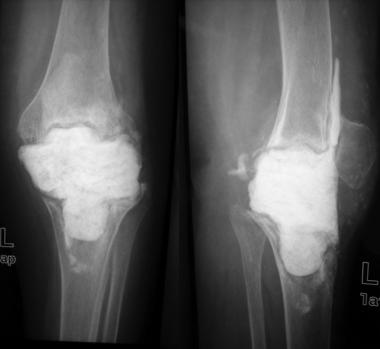 Radiograph after stage I revision.
Radiograph after stage I revision.
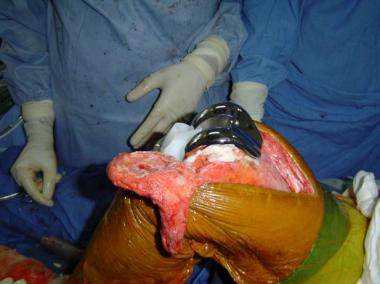 Stage II revision. Bone loss at femoral side; allograft used.
Stage II revision. Bone loss at femoral side; allograft used.
 Astemmed femoral prosthesis and allograft used for bone loss.
Astemmed femoral prosthesis and allograft used for bone loss.
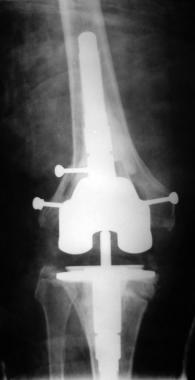 Final radiograph after stage II revision.
Final radiograph after stage II revision.
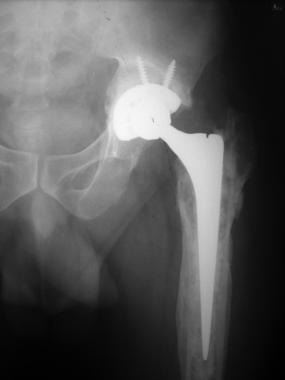 Final radiograph after stage II revision.
Final radiograph after stage II revision.
When exchange is being considered, many important factors must be taken into account. In particular, the host should be evaluated for any problems that may cause a recurrence of infection, including the following[28] :
The extent of disability after component removal, the virulence of the infecting organisms, the adequacy of débridement, and the degree to which infection is controlled at local and distant sites are some of the important variables affecting the outcome of an exchange procedure. The literature suggests that infections caused by gram-negative organisms are more difficult to treat than those caused by gram-positive isolates.[63, 64, 65, 66, 67]
Removal of components can be delayed until culture sensitivities are available, unless the patient is in a toxic state. All cement must be carefully removed; image intensification may be necessary to accomplish this. The difficulty of eradicating bacteria adhering to foreign bodies has been well described,[68] as has the need to remove all hardware and cement in the treatment of periprosthetic infections.[55, 57, 67, 69, 70, 71] Periprosthetic tissue should be obtained at the time of prosthesis removal for culture and histologic examination.
If a later exchange is planned, an antibiotic-impregnated spacer or antibiotic beads should be placed after removal of the components (see the image below). An antibiotic-impregnated spacer can be made from antibiotic-loaded cement in the operating room (OR). The spacer maintains limb length and soft-tissue tension and makes the definitive reimplantation procedure easier; however, there is a chance that it may fracture or dislocate.
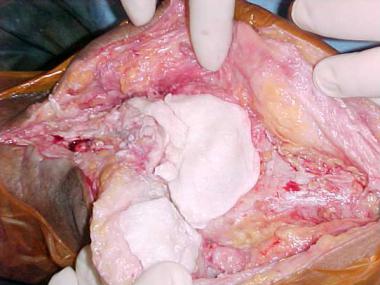 Cement spacer application.
Cement spacer application.
This device is a “dynamic” spacer, in that it allows movements and avoids muscle wasting. Initial results have been successful.[72] In place of a spacer, some authors prefer to use tobramycin beads, made with a mixture of 1.2 g of tobramycin and 20 g of polymethylmethacrylate (PMMA).[73] Antibiotics are also administered intravenously (IV) for 6 weeks, after which time a delayed exchange arthroplasty is performed.
One report described a two-stage exchange arthroplasty technique using an antibiotic-impregnated cement intramedullary nail, which can be easily prepared during surgery and which may provide additional stability to the knee and help it maintain its normal mechanical axis.[74] The advantages claimed for this technique include less pain between prosthesis removal and subsequent reimplantation, less soft-tissue contracture, less scar adhesion, easy removal of the cement intramedullary nail, and successful infection control.
The time interval between removal of the components and implantation of the revision prosthesis varies widely. Typically, the revision is performed 6-12 weeks after the removal of the prosthesis (first stage). The antibiotics should be discontinued 2 weeks before the definitive treatment. Multiple cultures of specimens should be obtained during the revision to confirm the eradication of the infection. The spacer or the tobramycin beads are removed from the joint at that time.
The definitive implants can be either cemented or cementless. Factors reported to be most important in deciding to insert the implant without cement are an age of less than 65 years and thinning of the cortical wall of the femur.[28] A thin cortical wall has been found to be associated with decreased shear strength at the bone-cement interface of revision prostheses.[75] When cemented implants are inserted, antibiotic-impregnated cement (eg, 1.2 g of tobramycin in 40 g of cement) should be used.
Whenever possible, bulky implants are avoided in the knee; a constrained prosthesis is associated with higher rates of infection. Bone defects left after implant removal may have to be treated with large structural allografts. Although some studies have reported increased infection rates with large allografts, the use of such grafts has increased.[76, 77]
A great deal of controversy exists regarding the relative advantages and disadvantages of direct exchange and delayed exchange, each of whih has specific merits and the limitations (see Table 3 below).
Table 3. Advantages and Disadvantages of Direct and Delayed Exchange (Open Table in a new window)
Direct Exchange Delayed Exchange AdvantagesGood results have been obtained with delayed-exchange arthroplasty in the treatment of chronic infections: the reported success rate is approximately 85%.[28, 38, 67, 78, 79, 80, 81]
The best choice of antibiotic for staphylococcal infection has been defined. Rifampin should be used against sensitive organisms, always in combination with another drug (preferably a quinolone) to prevent the emergence of resistance.[82, 83] Newer quinolones, such as moxifloxacin, levofloxacin, and gatifloxacin, have better activity in vitro against quinolone-susceptible staphylococci than ciprofloxacin, fleroxacin, and ofloxacin do.[29]
Other drugs tried in combination with rifampin include trimethoprim-sulfamethoxazole, minocycline, and fusidic acid. One study showed good results with the rifampin–fusidic acid combination in staphylococcal infections.[84]
Quinopristin-dalfopristin is active against Enterococcus faecium (including vancomycin-resistant enterococci [VRE]) and S aureus (including methicillin-resistant S aureus [MRSA]) but not against Enterococcus faecalis. In a study of 40 patients with orthopedic infections with MRSA treated with this combination, clinical success was reported in 78% and microbial eradication in 69%.[85]
Daptomycin is active against several gram-positive bacteria, including MRSA, vancomycin-resistant S aureus, and VRE.[86]
Linezolid has been used against MRSA, but long-term administration can lead to reversible optic neuropathy, myelosuppression, and irreversible peripheral neuropathy.[87, 88] Data regarding the combination of linezolid with rifampin are lacking. The use of rifampin may be a concern in countries where tuberculosis is endemic; it could help foster the development of resistance in tubercle bacilli.
Trampuz et al recommended antibiotic regimens for various microbial agents causing periprosthetic infections (see Table 4 below).[29]
Table 4. Antibiotic Regimens for Various Pathogens Causing Prosthetic Infections (Open Table in a new window)
Microorganism Antimicrobial Agent1 Dosage Route Staphylococcus aureus or coagulase-negative staphylococci Methicillin-susceptible RifampinThe use of antibiotic-impregnated cement has greatly altered the delivery of antibiotic to the infection site. The most commonly used antibiotics are tobramycin, vancomycin, and gentamicin. Some antibiotics (eg, lincomycin and tetracycline) are deactivated when mixed with cement. Rifampin forms a black, tacky composite and hence should not be used.
The recommended antibiotic doses vary, depending on whether the cement is being used as beads or spacers or for fixation of implants. For fixation of implants, 1 g of vancomycin or 1.2 g of gentamicin or tobramycin is the ideal dose; for use in spacers or beads, these doses can be doubled.
Although there are no strict guidelines regarding the optimal duration and route of antibiotic therapy, intravenous (IV) administration of antibiotics for 6 weeks in doses that achieve a bactericidal concentration of at least 1:8 have proven efficacious in reimplantation protocol studies. A higher drug concentration is needed for bacteria adhering to cement or polyethylene than for suspended bacteria.
Long-term oral antibiotic therapy may be considered in elderly patients who have a short life expectancy and from whom removal of the prosthesis may be extremely difficult. For such therapy to be a worthwhile option, the organism should be of low virulence and susceptible to an oral antibiotic, and the antibiotic should have no serious side effects. Treatment is individualized.
The disadvantages of long-term antibiotic suppression—namely, the emergence of multidrug resistant organisms, the formation of excessive scar tissue, and the progressive loss of bone stock—must be taken into account in the decision-making process.
Young patients with a high functional demand and a single joint involvement are ideal candidates for knee arthrodesis. Factors that rule out a revision procedure (eg, loss of extensor mechanism, poor soft-tissue cover, systemic immune compromise, and highly virulent organisms) also warrant a knee arthrodesis.
Relative contraindications for arthrodesis include bilateral knee disease, ipsilateral ankle or hip disease, severe segmental bone loss, and amputation in the contralateral extremity.
Excision or resection arthroplasty (the Girdlestone operation, as it is commonly referred to when done in the hip joint) is performed either as a definitive procedure or as a temporizing measure to buy time for infection control before reimplantation.
As a definitive procedure, excision hip arthroplasty is typically successful at controlling the infection, but many patients are unable to walk without assistive devices, and all have a limp. The patient is left with a painless unstable hip. Many patients are unwilling to accept the problems associated with excision arthroplasty and request reimplantation instead. The proximal femur is preserved as much as possible.
Current indications for excision arthroplasty in an infected knee are few but are considered to include inflammatory arthropathy. A study of 26 patients, 11 of whom had rheumatoid arthritis, showed that 89% were free of infection at an average of 5 years after excision arthroplasty.[89] Functional results were suboptimal: only 15 patients were able to walk independently, and all required walking aids. Only five patients had sufficient knee stability to walk without external support; eight required a knee-ankle-foot orthosis, and two used a splint.
Although excision knee arthroplasty usually results in satisfactory resolution of the infection, most patients experience some pain and knee instability and have a limited ability to walk.
Copyright © www.orthopaedics.win Bone Health All Rights Reserved