

Metal has been used extensively in the manufacturing of orthopedic implants in a multitude of different forms. Multiple different materials throughout history have been tested as replacements for bone. Materials as diverse as ivory, wood, rubber, acrylic, and Bakelite have been used in the manufacture of prosthetic implants.
The extensive use in modern times of metallic alloys is related to the availability and success at the beginning of the 20th century of several different alloys made of the noble metals. Implants made from iron, cobalt, chromium, titanium, and tantalum are commonly used. Clinical studies have demonstrated that alloys made from these metals can be used safely and effectively in the manufacturing of orthopedic implants that are left in vivo for extended periods. The mechanical, biologic, and physical properties of these materials play significant roles in the longevity of these implants.
Examples of metallic-alloy implants are shown below:
 Metallic alloys. Tantalum (left) and titanium (right) fiber mesh acetabular cups.
Metallic alloys. Tantalum (left) and titanium (right) fiber mesh acetabular cups.
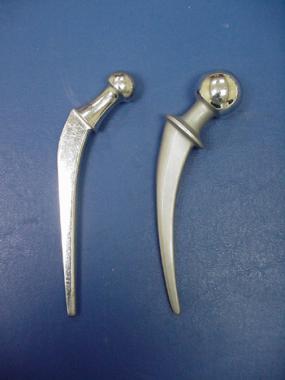 Metallic alloys. Stainless steel Charnley stem (left) and a cobalt-chromium Mueller (right).
Metallic alloys. Stainless steel Charnley stem (left) and a cobalt-chromium Mueller (right).
 Metallic alloys. Composite stems combine the physical properties of alloys with those of other biomaterials. Note, ceramic or metal femoral heads are used on composite hip stems because composites have relatively poor wear properties.
Metallic alloys. Composite stems combine the physical properties of alloys with those of other biomaterials. Note, ceramic or metal femoral heads are used on composite hip stems because composites have relatively poor wear properties.
Implants are made in 3 basic ways:
Alloys that provide for a long-term stable implant need to have a high level of corrosion resistance as well as certain mechanical properties (see Immune Response to Implants).
Dalury et al followed 96 patients for 5 years who had undergone total hip arthroplasty with single titanium stems. The average Harris Hip score was 96 points (range, 73-100 points) at final follow-up, and radiographically, all stems were ingrown. No stem had more than 3 mm of subsidence, and there were no leg-length discrepancies more than 5 mm. The authors concluded that the titanium stem is a versatile option for total hip arthroplasty.[1]
Grupp et al reported their experience regarding failed modular titanium neck adapters, in combination with a titanium alloy modular short hip stem, after hip arthroplasty, as a result of fretting or corrosion. They were then replaced by cobalt-chromium adapters. The authors noted that by the end of 2008, 1.4% (68 of approximately 5000) of the implanted titanium alloy neck adapters failed at an average of 2 years' time (0.7 to 4.0 years) postoperatively. The investigators concluded that failure of modular titanium alloy neck adapters can be initiated by surface micromotions due to surface contamination or highly loaded implant components. In the study, according to the authors, the patients at risk were men with an average weight over 100 kg. They added that with a cobalt-chromium neck, micromotions can be reduced by a factor of 3 and the incidence of fretting corrosion substantially lowered.[2]
NextAn element is considered metallic if a positive charge is demonstrated on an electrolysis test.[3] This test consists of dissolving the element in acid and running a current through the solution. When such elements are fully reduced, their metallic nature is recognized and they and their alloys are called metals; when oxidized, they can serve as ceramic materials.[4]
Metals have several properties that are specific to them, including malleability, which allows the shaping of metal into implants, and ductility, which refers to the ability to draw out metal in the shape of wire and is an important property in allowing the manufacture of intramedullary rods, screws, and long stems. By combining several metallic elements together in alloys, improved properties can be achieved beyond those of a single element. The alloys used in orthopedic surgery need to have certain specific properties. Because the alloy of the implant is bathed in body fluid, a low rate of corrosion and relative inertness are imperative in the material.
All metallic alloys have a modulus of elasticity significantly higher than that of bone. This mechanical incompatibility causes implants to be structurally stiffer than bones. Alloys with elastic moduli closer to bone may cause less stress shielding.
Different metals can form a battery effect when in solution in the body. The galvanic series provides electrochemical comparisons that allow for predictions of corrosion between 2 different metals when they are in physical contact in saline solution.[5] Galvanic corrosion occurs if stainless steel surgical wire is wrapped over a cobalt- or titanium-based alloy femoral component or if a cobalt-chromium femoral head is placed on a titanium alloy femoral stem, so this metal mismatch is not recommended. Cobalt- and titanium-based alloy components may be used in contact with each other, and stainless steel components, such as sutures, may be used with either if actual physical contact is avoided.
The introduction of steel plates for fracture treatment is credited to Sherman.[6] Surgical stainless steel alloys (316L) made with varying amounts of iron, chromium, and nickel are presently used in the manufacture of prostheses. The low carbon (L) in surgical stainless steel diminishes corrosion and decreases adverse tissue responses and metal allergies. While many implants are still manufactured from this excellent material, its use is relegated mainly to plates, screws, and intramedullary devices that are not meant to be weight bearing for an extended period. Fatigue failure and relatively high corrosion rates make it a poor candidate for the manufacture of modern joint replacement implants.[7]
Chromium-containing iron (and cobalt base) alloys have a chromium oxide–based surface that is a result of passivation or oxidation of the surface. The chromium oxide forms a very thin invisible shield that provides resistance to biodegradation. Because this oxide layer slowly dissolves in vivo, these alloys have a relatively high rate of corrosion. This is evident as a propensity toward both fretting and crevice corrosion, which limits the possibility for biologic fixation or for the manufacture of modular implants.
Venable and Stuck discovered the battery effects of metals in the body through their testing of the electrolytic effects of various metals on surrounding tissue and bone.[3] These tests demonstrated the low level of corrosion of the cobalt-based alloy vitallium. Alloys made of cobalt, chromium, and molybdenum can be used in various different porous forms to allow for biologic fixation by ingrowth. These alloys are among the least ductile when compared to either iron- or titanium-based alloys, making manufacture of these intramedullary rods and spinal instrumentation more difficult. These alloys have some of the highest moduli of elasticity observed in orthopedic implants, and as a result, this was a factor in the stress shielding and thigh pain observed in the first generation of biologically fixed femoral hip implants made with cobalt alloys.[8]
These alloys are well suited for the production of implants that are designed to replace bone and to be load bearing for an extended period, if not permanently.
The Austin Moore prosthesis and the Thompson prosthesis were manufactured from the cobalt-based alloys. The first-generation biologically fixed implants (ie, porous-coated anatomic [PCA] and anatomic medullary locking [AML] implants) were manufactured of this material. Numerous modern prostheses are still manufactured from this excellent alloy and are used in both cemented and porous forms for hip and knee replacement.
In 1951, Levanthal introduced titanium as a metal for surgery.[9] Titanium-based alloys have excellent properties for use in porous forms for biologic fixation of prostheses. The most common is Ti-6 aluminum Ti-4 vanadium (Ti6Al4V), but other more modern alloys are coming into use. Because of the lower moduli of elasticity than cobalt-based alloys or surgical stainless steel, titanium-based alloys have not been found to be as reliable a material when used as a cemented hip replacement. Moreover, its use in total knee replacements has been limited to the nonarticulating parts of the tibial component because of significant wear observed in femoral heads.[10]
Titanium's high level of biocompatibility, low level of corrosion, and modulus of elasticity closer to that of bone allow for its use in numerous porous implants that have yielded excellent long-term results. The low level of corrosion allows for the construction of modular implants that saves in inventory and allows for more precise implant fit.[11]
Current use in various forms is in the production of fracture plates and intramedullary rods and in the production of both femoral and acetabular implants designed for bone ingrowth. Fracture fixation components fabricated from titanium-based alloys are also used preferentially when the implant site is known to be infected or when postoperative shadow-free imaging is desired.
Industry has been modifying the surface area of titanium implants with many proprietary coatings. Attempts at mimicking the microscopic structure of cancellous bone has been extremely effective in increasing the scratch fit noted with these implants. Time will tell whether or not the longevity of these coatings will result in improved long-term ingrowth of components.
Tantalum is also remarkably resistant to corrosion and has been used as an ingredient in super alloys, principally in aircraft engines and spacecraft, although 50% of current use is in the form of powder metal for the manufacture of transistors and capacitors. Tantalum can be fabricated in a highly porous form, which has a modulus of elasticity closer to that of bone than stainless steel or the cobalt-based alloys. Tantalum balls have been used in studies that have required bone markers; however, it has not been used in the manufacture of implants until recently. Because of its remarkable resistance to corrosion, tantalum is well suited to a biologic ingrowth setting.
Recent use of tantalum has been in the form of a honeycombed structure that is extremely porous and conducive to bone ingrowth. It is currently available in several forms for bridging bone defects, but its use in the manufacture of femoral stems has yet to occur. Tantalum appears to be a promising metal for use in acetabular reconstruction, but long-term studies need to be conducted.[12, 13, 11, 14, 15]
The combination of metallic alloys with other biomaterials can result in implants with improved mechanical and physical properties. Current attempts in designing composite implants have not yielded highly successful results; however, the future possibilities for improvement are promising.
Different alloys demonstrate different rates of wear. The hardness of an alloy and the smoothness of the bearing surfaces determine its relative rate of wear. Cobalt-chromium-molybdenum alloys and alloys made of stainless steel are more wear resistant than titanium or titanium-based alloys. When breakdown with titanium-based alloys occurs, it is often observed as black areas within the tissues.
Metallic ion release occurs in vivo, and numerous studies demonstrate soluble and precipitated corrosion products, as well as metallic wear debris, in the liver, spleen, lungs, and even remote bone marrow of the iliac crest. The constant motion of the metal-on-metal prosthesis causes a wearing away of the passivated surface and an increase in metallic ion release. The recent interest in metal-on-metal prostheses raises questions of biocompatibility and possible carcinogenic effects that these metallic ions can cause.[16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28]
Recently, several metal-on-metal prostheses have been recalled, and new concerns have arisen about the long-term safety of metal-on-metal prostheses. In April 2010, the United kingdoms Medicines and Healthcare Products Regulatory Agency issued a medical device alert on metal-on-metal hip replacements. Recommendations have included specific blood tests and imaging for patients with painful metal-on-metal hip replacements. Metal ion testing and evaluation for effects of metal debris such as possible local nerve palsy, local swelling, and joint dislocation or subluxation should be considered by orthopedic surgeons treating patients with metal-on-metal prostheses.
Hopefully, further developments in metallurgy will allow for the development of new alloys that, when compared to current alloys, will have better mechanical and physical properties yielding better long-term results with implants.
The concurrent developments in other biomaterials, such as ceramics, and newer modified polyethylenes, such as cross-linked polyethylene, hopefully will result in improvements in longevity of total joint replacements either with the success of alternative bearing surfaces or with the use of composite materials. The total joint replacement that will last the life of the patient may be a reality one day.[29, 30]
Copyright © www.orthopaedics.win Bone Health All Rights Reserved