

Immune response to implants is commonly reported in the literature and can include hypersensitivity related to pacemakers, dental implants, and orthopedic hardware.[1] Furthermore, as many as 13% of people are sensitive to nickel, cobalt, or chromium.[1, 2, 3, 4, 5, 6, 7, 8, 9] The development of metal sensitivity after implantation of orthopedic hardware is common.[1, 10, 11, 12]
Metal sensitivity is also correlated with osteolysis and aseptic loosening of implanted metal hardware.[5, 10, 12, 13, 14, 15, 16, 17, 18] However, statistical reviews of cases involving adverse reactions after implantation of metal hardware demonstrate that metal sensitivity can be proven causative in fewer than 0.1% of cases in which sensitivity reactions exist.[1, 19, 20, 21, 22]
According to Huber et al, the presence of corrosion products and a hypersensitivity reaction in patients suggests that there is a relation between corrosion and implant-related hypersensitivity. In 11 cases in which periprosthetic tissue contained corrosive elements (solid chromium orthophosphate corrosion products) after aseptic loosening of articular implants, immune-response tissue reactions were identified in all 11 cases.[23]
The issue of the clinical significance of sensitization to implanted metals has long been debated in the literature. Although some studies have found sensitization to metal implants to be prevalent,[1, 10, 11, 12] others have taken an opposing viewpoint, concluding not only that hypersensitivity fails to develop[24] but also that patients with metal hypersensitivity prior to implantation actually become desensitized and anergic after implantation.[25] Some authors have further suggested that metal hypersensitivity may be associated with bone loss and aseptic loosening of implanted devices,[5, 11, 14] whereas others have argued that even if a metal allergy exists, no adverse effects occur.[13, 24, 26, 27]
These contradicting studies make it difficult for the orthopedic surgeon to make the diagnosis of symptomatic metal allergy with confidence. The confusion could be the result of the presence of different metals in the implants, different manufacturing methods, small numbers of patients in the studies, and difficulty in achieving adequate diagnosis.
Although prescreening all patients for metal hypersensitivity may be costly, with dubious clinical relevance, various specific laboratory tests, including the lymphokine migration inhibition factor test, appear to be confirmatory. Because this is a diagnosis of exclusion, cases are difficult to identify because the signs and symptoms are very similar to other more frequent causes. In the rare case in which a patient displays symptoms such as recurrent pain and aseptic loosening related to implanted hardware, the differential diagnosis of metal hypersensitivity should be considered.
More research is clearly needed. In the meantime, the orthopedic surgeon must be aware of the potential problem, but the diagnosis must be made with caution. Infection, nonunion, aseptic loosening, other inflammatory conditions, mechanical failure of the implant, and malalignment issues must be excluded first before the problem is assumed to be an allergic reaction. Once other more common causes of implant failure have been excluded, the possibility of allergic reaction to the metal must be considered, evaluated, and treated.[28]
For patient education resources, see Total Hip Replacement and Knee Joint Replacement.
NextThe presenting signs and symptoms of a nickel hypersensitivity to an implanted orthopedic device are variable but usually consist of the expected complaints of a patient with hardware failure.
Patients with joint replacements have symptoms of loosening, including pain and instability. (For example, with a total hip replacement, the patient often has groin pain radiating to the medial thigh.) Patients with hardware for fractures have symptoms of nonunion, including pain and motion at the fracture site. Local inflammatory symptoms similar to the symptoms of infection are also possible, including warmth, erythema, and swelling over the implant, although systemic complaints such as fever are unlikely. A skin rash may develop over the metal device but is not always present.[29, 30, 31]
Osteolysis due to implant loosening is always in the differential diagnosis. Fujishiro et al identified an association between the extent of inflammation and the amount of visible metal particles and concluded that this relation implied the occurrence of an immune response to the metal. However, the typical morphologic features of an immune inflammatory reaction, including loss of the surface synovial lining, fibrin deposits, and lymphocytes in diffuse and perivascular distributions was not consistently present.[32]
More likely, the mechanism of osteolysis is primarily a local reaction to particulate debris,[33] which leads to a cascade of cellular reactions (including activation of monocytes/macrophages, phagocytosis, release of cytokines) that eventually lead to increased osteoclastic activity around the prosthesis. Osteolysis is a reaction to local irritation, not an immune hypersensitivity response.[34]
The causes of these different patterns of inflammation are unknown, but the association between the extent of inflammation and visible metal particles (but not zirconium particles) supports the concept of an immune reaction to metal, and it illustrates that the process is not specific to metal-on-metal constructs.
Currently, blood tests are rarely performed in the workup of immune responses to implants. Generally, white blood cell counts and other assessments of inflammatory mediators (eg, platelet counts, C-reactive protein levels, and sedimentation rates) are not elevated or are only minimally elevated, and they are not specific or reliable enough to help in the diagnosis.
Careful radiographic assessment of the implant is required. Radiolucencies around the hardware, screw migration, and change in position of the implant imply loosening that could be due to hypersensitivity to the metal. Cystic changes, as seen in osteolysis, may be seen (see the image below). Computed tomography (CT) and magnetic resonance imaging (MRI) are not especially helpful. A bone scan may be positive, but the findings are nonspecific.
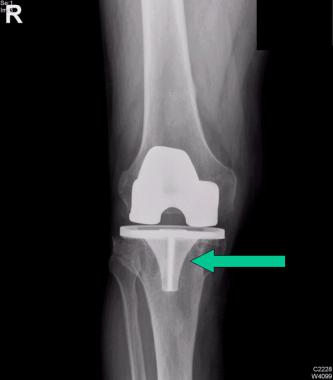 Osteolysis around a total knee implant.
Osteolysis around a total knee implant.
Traditionally, skin patch testing has been the standard screening test for metal hypersensitivity. This method is limited by the fact that a positive result is not indicative of a true hypersensitivity but must be considered in the context of a patient's medical history and physical findings.[35, 36] A routine skin test reveals a prevalence of metal sensitivity of 0.2% for chromium, 1.3% for nickel, and 1.8% for cobalt. After placement of metal implants, sensitization (change from negative to positive) occurs in 2.7% for chromium, 3.8% for nickel, and 3.8% for cobalt. Desensitization (change from positive to negative) occurs in 0% for chromium, 2.1% for nickel, and 3.8% for cobalt.[37]
Many patients with implanted metal hardware have positive skin test results for those metals but are completely asymptomatic.[13, 24] If the skin patch test finding is positive, the patient can be designated as allergic. However, the clinical significance of the allergy is controversial. Most allergy skin patch tests that show skin reactivity have no clinical implications.
The causes of these skin immunologic reactions are unclear.[1, 36] It is thought that antigen-presenting cells that are localized to the skin (dendrite cells) may handle antigens differently than the antigen-presenting cells that are systemic (macrophages and monocytes). Therefore, many people who have skin reactivity to metals may never develop any reactivity at the site of prosthesis composed of that metal. Skin test results may not return to normal after metal removal.[16]
Conclusions based on skin patch testing should therefore be made with caution and only assumed valid if the whole clinical picture supports the finding of symptoms related to metal allergy. Preoperative skin patch testing is not typically recommended unless there is a strong suggestion of established sensitivity by history, because of a slight chance of sensitization and the high cost/low yield results expected.
Tests that may be more specific include the lymphocyte transformation test (LTT) and the lymphokine migration inhibition factor (MIF) test, which have been used to help diagnose metal hypersensitivity.
In vitro lymphicyte proliferation testing is perhaps the most prevalent method after patch testing for assessing hypersensitivities. It involves measuring the proliferative response of lymphocytes following activation. A radioactive marker is added to lymphocytes along with the desired challenge agent. The incorporation of radioactive 3H-thymidine marker into cellular DNA on division facilitates quantification of a proliferation response through the measurement of amassed radioactivity after a period of 3-6 days. At day 6, 3H-thymidine uptake is measured by using liquid scintillation. The proliferation factor or stimulation index is calculated by using measured radiation counts per minute (cpm), as follows:
Proliferation factor = (mean cpm with treatment)/(mean cpm without treatment).
The use of proliferation testing in the assessment of metal sensitivity has been well established as a method for testing metal sensitivity in a variety of clinical settings.[38, 39, 40, 41, 42, 43] The technical sophistication and high expense of the LTT for implant-related metal sensitivity has limited its use; therefore, few conclusions can be drawn.[44, 45, 46] These few investigations using the LTT report that increased rates of metal sensitivity can be detected above that determined by dermal patch testing.[44, 46, 47] Such reports seem to indicate that the LTT, compared with dermal patch testing, may be equally or better suited for the testing of implant-related sensitivity.[39, 40, 41, 42, 43, 44, 45, 46, 48]
Another in vitro test that has shown promise in diagnosing metal hypersensitivity involved the use of MIF. MIF acts to prevent lymphocytes from leaving a site where foreign antigens are present. This test selectively detects lymphokine MIF, which, when present, does indicate an active immune response and metal sensitivity.[26]
The test is performed by obtaining a blood sample and isolating the lymphocytes. The lymphocytes are then mixed with solutions of specific metal ions, such as nickel, chromium, cobalt, or titanium. The test result is considered positive if the lymphocytes stay in the metal ion solution, indicating a cellular reaction to the metal dissolved in that solution. (In a positive result, no migration occurs.) The test result is considered negative if the lymphocytes migrate away from the particular metal ion solution, indicating that they are not reacting to the dissolved metal.[1]
Studies reveal that positive MIF test results to metals implanted in an orthopedic patient are well correlated with pain, swelling, and dermatologic reactions over that area. Furthermore, after the implanted materials are removed, these signs and symptoms improve, and the MIF test result returns to normal.[16] The lymphokine MIF is the most useful clinical test for diagnosis of hypersensitivity reaction to orthopedic implants (see the image below).[49] Cement wear particles are immunologically inert and have specifically been found not to cause a lymphocyte response in vitro[50] ; thus, the lymphokine MIF result should be negative in osteolysis.
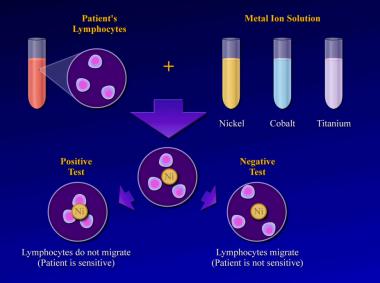 Lymphokine migration test.
Lymphokine migration test.
Another important factor to consider in the biologic response to orthopedic implants is metal ion exposure and release. Implants from different manufacturers have varying metal compositions (see the image below). For example, the nickel content in stainless steel may vary in the range of 9-15.5%, whereas in cobalt-base alloys, the nickel content is usually specified to be no greater than 1% (<0.2% in actual practice) and essentially 0% for titanium.
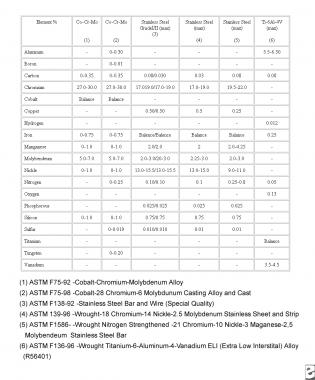 Composition of common metal alloys used in orthopedic implants.
Composition of common metal alloys used in orthopedic implants.
In addition to the percentage of a particular metal contained within an alloy, the nature of the alloy and local exposure of the implant are important. Alloys are graded on a scale that measures their metal ion release rate. For example, implant grade 316L (low carbon) stainless steel releases far less nickel than low-grade stainless steel suture. Local exposure of metallic surfaces also affects ion release and can be a factor in the development of hypersensitivity reactions secondary to an implant.
In a prospective cohort study that included 597 patients with metal-on-metal hip resurfacing and total hip prostheses, Hart et al found that elevated blood levels of metal (ie, chromium and cobalt) ions were associated with an increased risk of implant failure.[51]
Implant properties may alter the amount of surface area available for metal ion release. Plasma-spray coatings and grouting agents limit exposure and decrease ion release from the implant. Roughened, grit-blasted, or grooved surfaces increase the surface area available for ion release from the implant and thereby increase the local levels of dissolved metal.[8]
Taking these factors into consideration, many of the manufacturers of these alloys and implants are striving to make them as resistant to breakdown as possible in the hope that by limiting the quantity of ions released, it may be possible to decrease the rate of sensitization.[1, 52]
The usual course of events in patients demonstrating true postimplantation metal hypersensitivity is such that symptoms develop over months to years; this may be long after the device has accomplished the goal of fracture stability.[16] Gradual development of skin changes, pain, tenderness, and swelling over the area of the implanted hardware may be coupled with evidence of loosening of a previously stable implant.[16] Acute symptoms in patients with multipart devices may be associated with periods of increased activity.[53]
No medical treatment is available. Analgesic pain medicines, including nonsteroidal anti-inflammatory medications, may control symptoms but do not alter the underlying pathology. Surgical treatment includes either (a) revision (obviously with an implant with a different metal composition) for a joint replacement prosthesis or a fracture implant that is still necessary for fracture stability or (b) removal for an implant that is no longer necessary.
A 71-year-old woman had a right intertrochanteric hip fracture and underwent open reduction and internal fixation (ORIF) by using a standard stainless steel hip fracture implant (Synthes DHS; Paoli, PA). Postoperatively, the patient did well, with evidence of fracture healing, full weightbearing, and full range of motion by 3 months after surgery.
Approximately 6 months later, the patient began to complain of right hip pain laterally over the area of the implanted hardware. The fracture was radiologically healed, but because of the patient's unbearable pain, a technetium bone scan and tomogram of the area were obtained. The results demonstrated increased uptake and lucency around the lag screw, indicative of hardware loosening.
The patient underwent debridement, hardware exchange, and an iliac crest bone graft. Intraoperative cultures were obtained that all proved negative for an infectious cause. Again, 4 months postoperatively, the patient began to complain of similar right hip pain, though imaging showed good bone graft incorporation and fracture healing (see the image below).
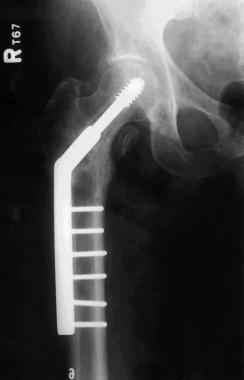 Case example. Second stainless steel implant in the patient's right hip. Image shows a healed fracture but failing hardware.
Case example. Second stainless steel implant in the patient's right hip. Image shows a healed fracture but failing hardware.
A course of anti-inflammatory medicines and steroid injections to the region relieved pain only briefly. Hardware removal was performed 10 months after hardware exchange (see the image below). The patient's symptoms resolved shortly thereafter.
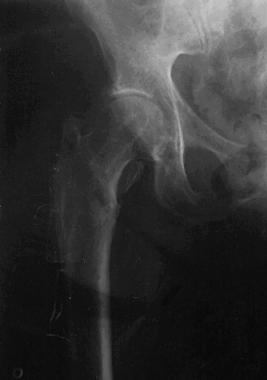 Case example. Image shows the right hip after the hardware was removed.
Case example. Image shows the right hip after the hardware was removed.
Four years later, the patient had an intertrochanteric fracture of the contralateral left hip and again underwent ORIF with a stainless steel device (Synthes DHS) (see the image below). She was recovering well with signs of fracture healing until 3 months after surgery, when she began to experience pain over the implanted hardware. Over the ensuing 3 months, the patient's pain increased to an unbearable level.
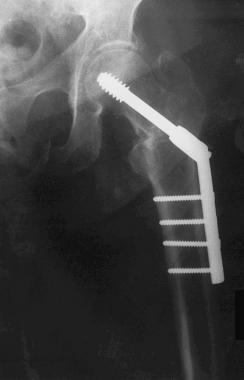 Case example. First stainless steel implant in the left hip.
Case example. First stainless steel implant in the left hip.
Radiographs demonstrated loosening and cut out of the hip lag screw, but the fracture was healing (see the image below). Accordingly, the patient underwent hardware removal 6 months after the initial implantation. At the time of the operation, a collection of serous fluid was noted around the implanted hardware, but no other clinical evidence of infection was observed. The hardware was not loose, and the fracture was stable and clinically healed. Irrigation and debridement were performed, and the patient was treated with intravenous antibiotics until intraoperative cultures proved to be negative.
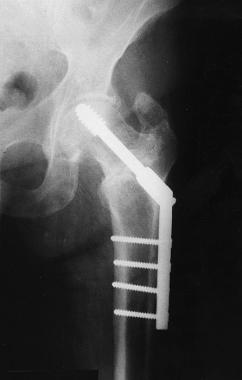 Case example. Image shows the failed stainless steel implant in the patient's left hip.
Case example. Image shows the failed stainless steel implant in the patient's left hip.
A short time later, the patient had a fracture of the femoral neck during therapy. Total arthroplasty of the left hip was recommended, but after consideration of her past orthopedic history, the patient was first referred to an allergist for metal allergy patch testing. The results were positive for an allergy to nickel sulfate (with >20 mm of erythema noted) but negative for chromium and cobalt. Given the false-positive results of skin patch testing, blood samples were sent for the lymphokine migration inhibition factor (MIF) test, which later confirmed nickel hypersensitivity (personal communication, Katharine Merritt, PhD, US Food and Drug Administration Office of Science and Technology).
The patient underwent left total hip arthroplasty by using a cementless titanium implant with a ceramic head (see the first image below). At 4-year follow-up, she had no further complaints or problems (see the second image below).
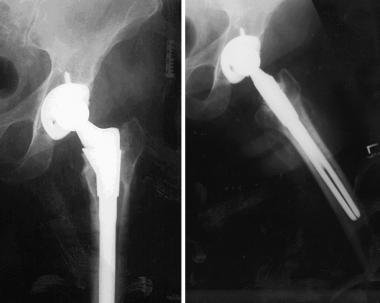 Case example. Image shows successful titanium total hip implant in the left hip.
Case example. Image shows successful titanium total hip implant in the left hip.
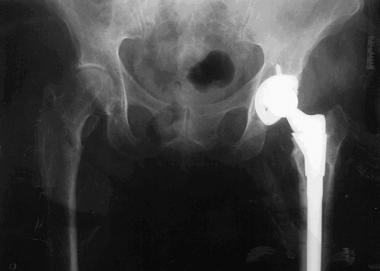 Case example. Final follow-up image after successful total hip replacement of the left hip.
Case example. Final follow-up image after successful total hip replacement of the left hip.
In retrospect, this example presents a strong possibility of a true metal hypersensitivity reaction. However, because such a reaction is a diagnosis of exclusion, definitive proof is difficult to achieve.
This patient received three different stainless steel devices at two different sites. At the first site (right hip), complete healing occurred, and the patient remained asymptomatic after the device was removed. At the second (left hip), again complete healing occurred and remained asymptomatic since a titanium device was implanted. The patient had positive nickel sensitivity, as shown on both skin patch testing and lymphokine MIF testing, and negative culture results with no clinical evidence of infection. It is likely that she did have a true metal sensitivity reaction causing clinical failure of hardware and disabling pain.
Copyright © www.orthopaedics.win Bone Health All Rights Reserved